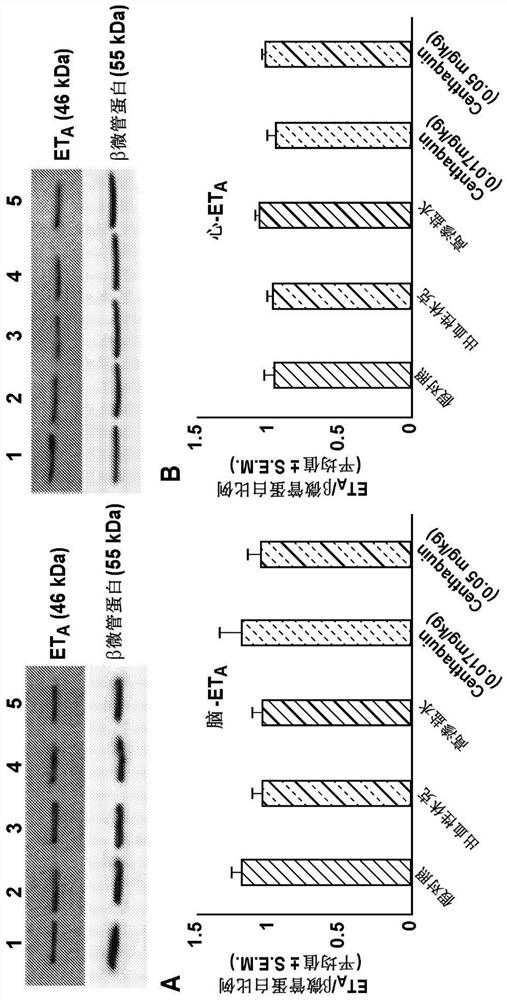
Alterations in endothelin receptors following hemorrhage and resuscitation by centhaquin
A technology of endothelin and receptor agonist, which is applied in cardiovascular system diseases, vector-borne diseases, and medical preparations containing active ingredients, etc. It can solve the problem of blood perfusion loss, hypothermia, immunosuppression, multiple organ failure and death in patients And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] method
[0079] Animals Prior to use, Sprague-Dawley male rats (340 to 380 g) (Envigo, Indianapolis, IN) were incubated at controlled temperature (23±1° C.), humidity (50±10%) and light (6:00 AM to 6 :00PM) feeding. Food and water are provided continuously. Animal care and use for experimental procedures were approved by the Midwestern University Institutional Animal Care and Use Committee. All anesthesia and surgical procedures were in accordance with guidelines established by the Animal Care Committee.
[0080] Pharmaceuticals and Chemicals Centhaquin citrate (PMZ-2010) was synthesized at Pharmazz India Private Limited, Greater Noida, India. Using urethane (urethane) (Sigma-Aldrich StLouis, MO, USA), hypertonic saline injection USP (Hospira, Inc, Lake forest IL, USA), and heparin sodium injection (APP Pharmaceuticals, LLC, Schaumburg , IL, USA). Endothelin-1 enzyme immunoassay kit (catalog number 900-020A, Assay Designs, Inc., Ann Arbor, MI, USA), IL-6 ELISA kit ...
Embodiment 2
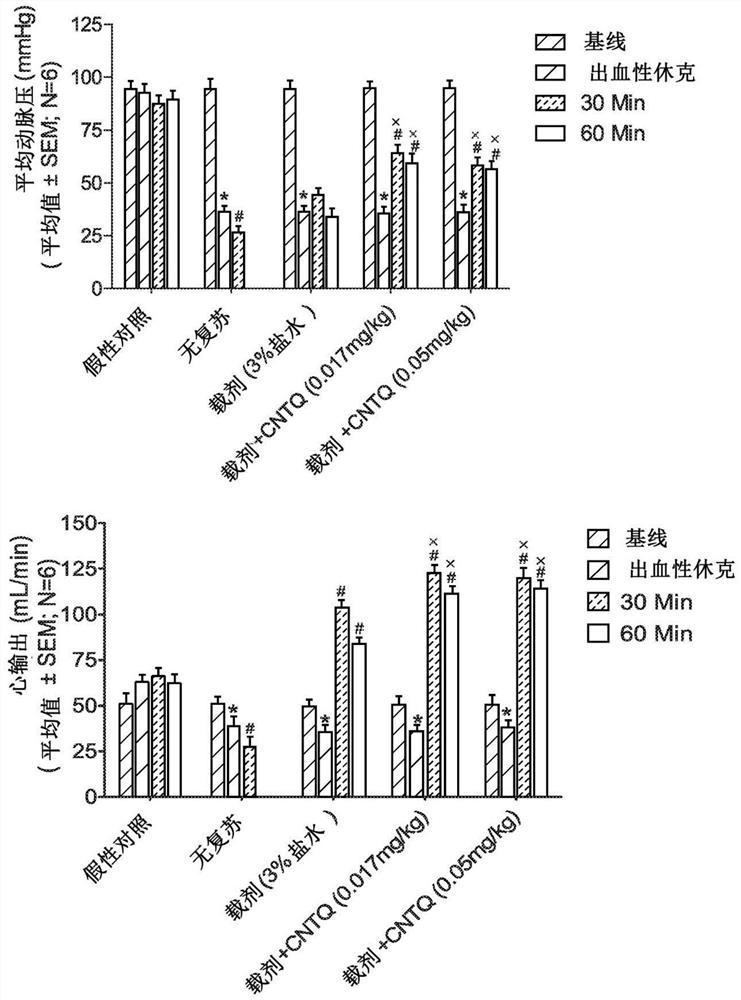
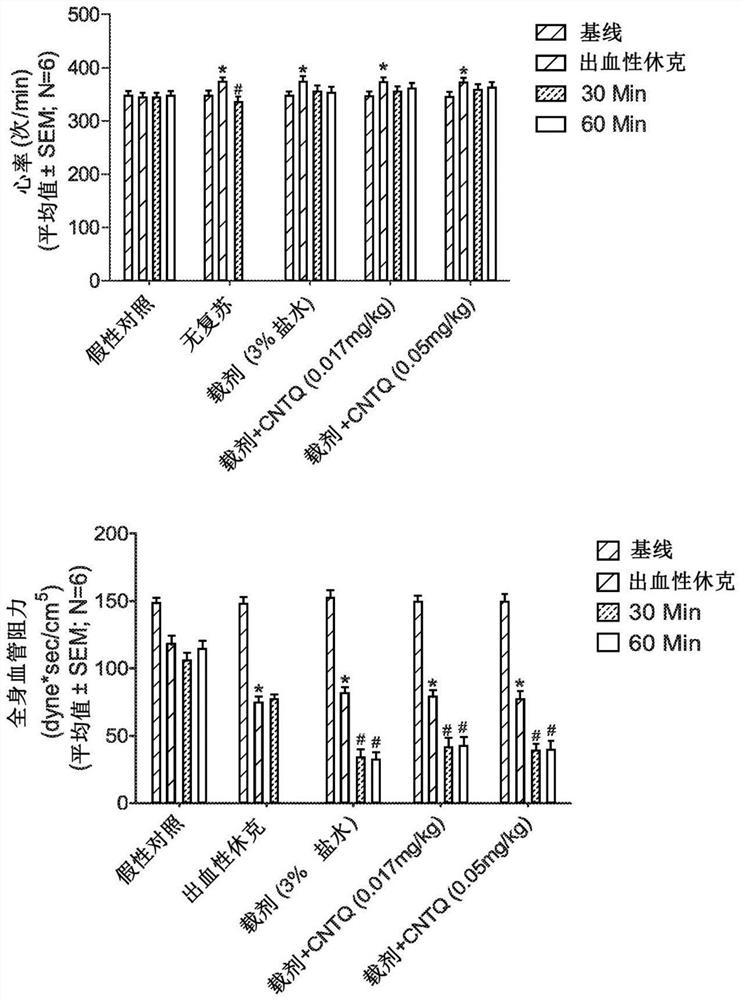
[0115] The following example identifies low doses of centhaquin citrate (2-[2-(4-(3-methylphenyl)-1-piperazinyl)]ethyl-quinoline) citrate in hemorrhagic shock Salt significantly lowers blood lactate and increases mean arterial pressure (MAP), pulse blood pressure (PP), and cardiac output (CO) (Gulati et al., 2012; Gulati et al., 2013; Lavhale et al., 2013; Papapanagiotou et al., 2016). A comparative study between centhaquin and existing resuscitation agents, which fall into 3 different classes: (a) fluids, such as lactated Ringer's, hypertonic saline; (b) adrenergic agents, such as norepinephrine prime, and (c) fresh blood. Our results using (i) a rat model of constant pressure hemorrhage, (ii) a rabbit model of uncontrolled traumatic hemorrhage and (iii) a pig model of massive hemorrhage showed that centhaquin was very effective in reducing mortality after hypovolemic shock Effective (Gulati et al., 2012; Gulati et al., 2013; Lavhale et al., 2013; Papapanagiotou et al., 2016...
Embodiment 3
[0134]Conducting a prospective multicenter randomized double-blind parallel phase II study (CTRI / 2017 / 03 / 008184) of PMZ-2010 (centhaquin) as a resuscitative agent in hypovolemic shock due to excessive bleeding compared with saline . All subjects will receive standard shock treatment (the type of treatment and care that will be provided by the registry). Patients were randomly assigned to the control group (N=7) who received standard care and saline, or the PMZ-2010 group (N=12) who received standard care and PMZ-2010. The interim analysis revealed comparable demographics of patients in the two groups (Table 2).
[0135] Table 2. An interim analysis showing comparable demographics of patients in the two arms of the phase II study.
[0136]
[0137] The study drug, PMZ-2010, met its primary endpoint for safety and no adverse events were reported. Treatment required fewer doses in PMZ-2010 than in controls (Table 3). Of the total hospital stay, patients treated with PMZ-20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com