A chimeric antigen receptor, chimeric antigen receptor umbilical cord blood nucleated cells and application thereof
A chimeric antigen receptor and nucleated cell technology, applied in the field of cord blood nucleated cells and their preparation, can solve the problem that the killing effect of solid tumors cannot meet the expected requirements, and achieve the effect of strong targeting and killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Design and Construction of Chimeric Antigen Receptor and Its Carrier Plasmid
[0044] 1. CAR design
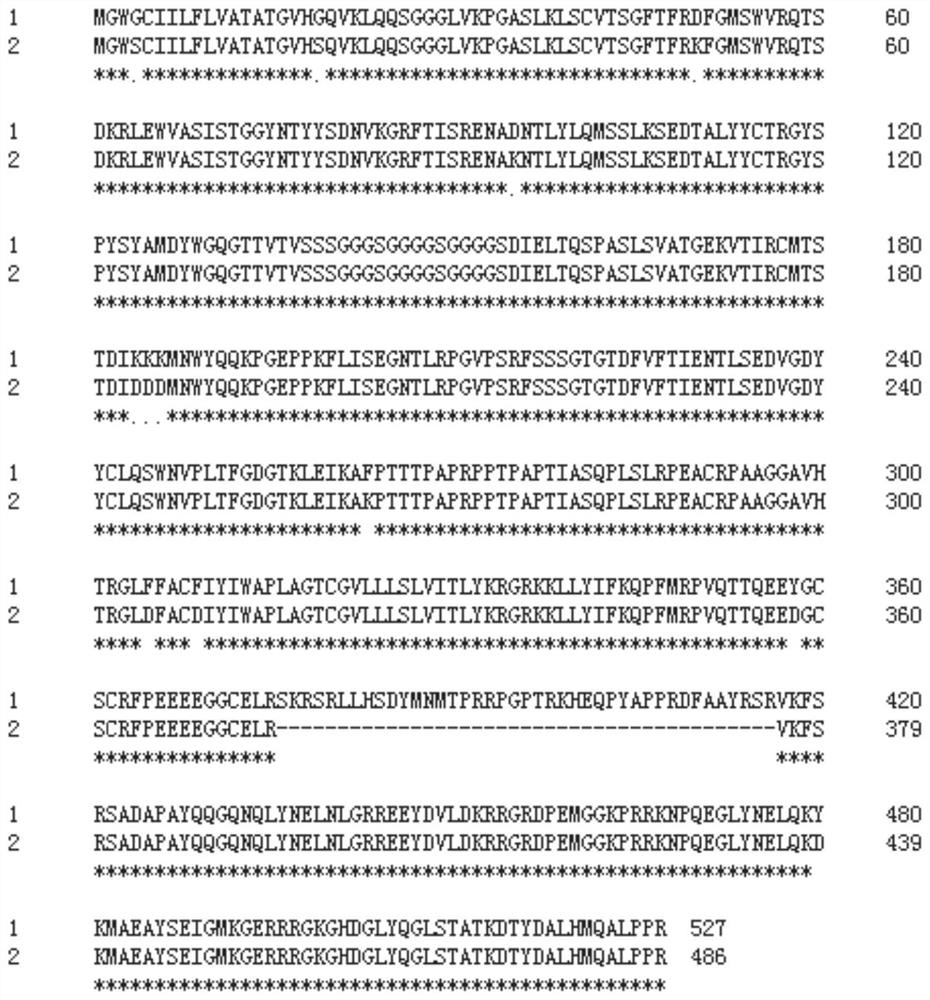
[0045] The chimeric antigen receptor (CAR) of the present invention comprises an antigen recognition domain, a transmembrane domain and an intracellular signaling domain of the human antibody EGFRvIII, and its amino acid sequence is shown in SEQ ID NO: 1, through patent CN105384826A The CAR mutation was obtained and achieved unexpected effects.
[0046] Chimeric antigen receptors are artificially constructed fusion proteins or polypeptides that exploit the antigen-binding properties of monoclonal antibodies to alter the specificity and reactivity of T cells to selected targets in a non-MHC-restricted manner, thereby bypassing the barriers of tumor escape. mechanism.
[0047] The CAR of the present invention has antigen specificity for epidermal growth factor receptor variant III (EGFRvIII). EGFRvIII, a variant of the epidermal growth factor receptor (EGFR),...
Embodiment 2
[0062] Example 2 Lentivirus transfection of umbilical cord blood nucleated cells
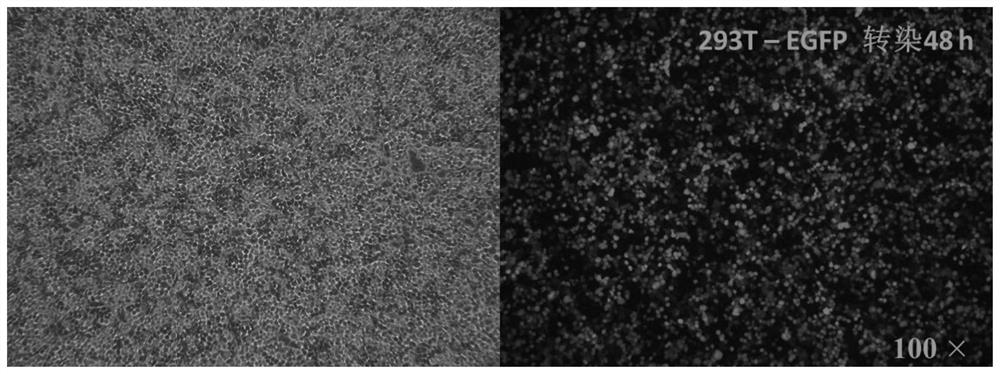
[0063] Isolation of nucleated cells from healthy human cord blood by gradient density centrifugation. Take 1×10 6 The cell / mL density was added to the T lymphocyte medium GT-T551 (Takara) containing 300IU / mL IL-2 (Beijing Shuanglu Pharmaceutical Co., Ltd.) for culture, and 5% of the same cord blood plasma was added, and 80ng / mL CD3mAb (Shanghai Weike Biotechnology Co., Ltd.) activated T cells and stimulated culture for 48 hours, then infected umbilical cord blood nucleated cells with the recombinant lentivirus of Example 1 at MOI=5, and the cells after infection were changed the next day, every 2-3 days passaging.
[0064] The cells are harvested after about 10 days of culture, and the cells can be expanded by 30-80 times, and the cells can still be expanded after reactivation. Under the fluorescent microscope, it can be observed that the isotype control cells highly express green fluorescent...
experiment example 1
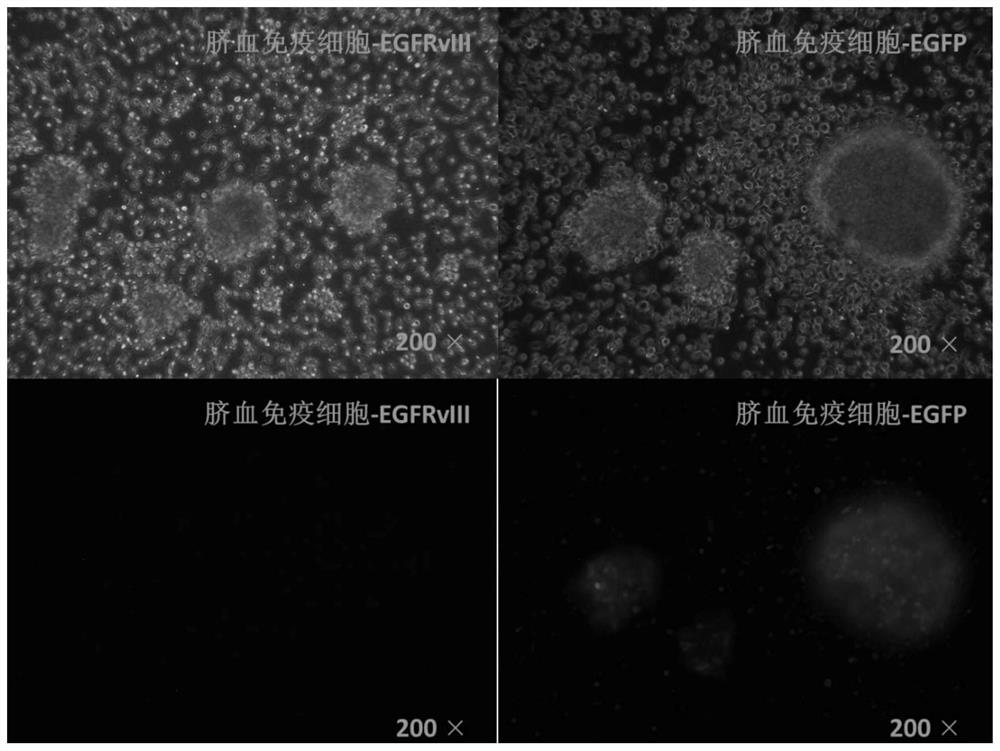
[0067] Experimental example 1 in vitro tumor cell killing
[0068] The chimeric antigen receptor cord blood nucleated cells prepared in Example 1 and Example 2 of the present invention, the chimeric antigen receptor cord blood nucleated cells prepared in Comparative Example 1, and the unprepared cord blood nucleated cells ( Negative control group) in vitro tumor cell killing effect was compared. The specific steps are as follows: 1×10 5 Effector cells (chimeric antigen receptor umbilical blood nucleated cells prepared in the present invention, chimeric antigen receptor umbilical blood nucleated cells prepared in Comparative Example 1, EGFP control cells, unprepared umbilical cord blood nucleated cells) and Target cells (U251 human glioblastoma cells) at 37°C, 5% CO 2 The co-cultivation was carried out under the conditions, and the cells were collected 15-18 hours after the culture, and the cell killing was detected. The result is as Figure 5 As shown, it is shown that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com