Synthesis method of alpha-cyano quaternary carbon substituted tetrahydroisoquinoline compound
A technology of tetrahydroisoquinoline and cyano quaternary carbon, which is applied in the synthesis of α-cyano quaternary carbon substituted tetrahydroisoquinoline compounds and the field of synthesis of isoquinoline compounds, which can solve the problem of narrow substrate application range, The reaction process is complicated, the reaction conditions are harsh, etc., and the reaction operation is convenient and practical, the activity is high, and the reaction conditions are mild.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 A synthetic method of 1-phenyl-1-cyano-2-methyl-1,2,3,4-tetrahydroisoquinoline
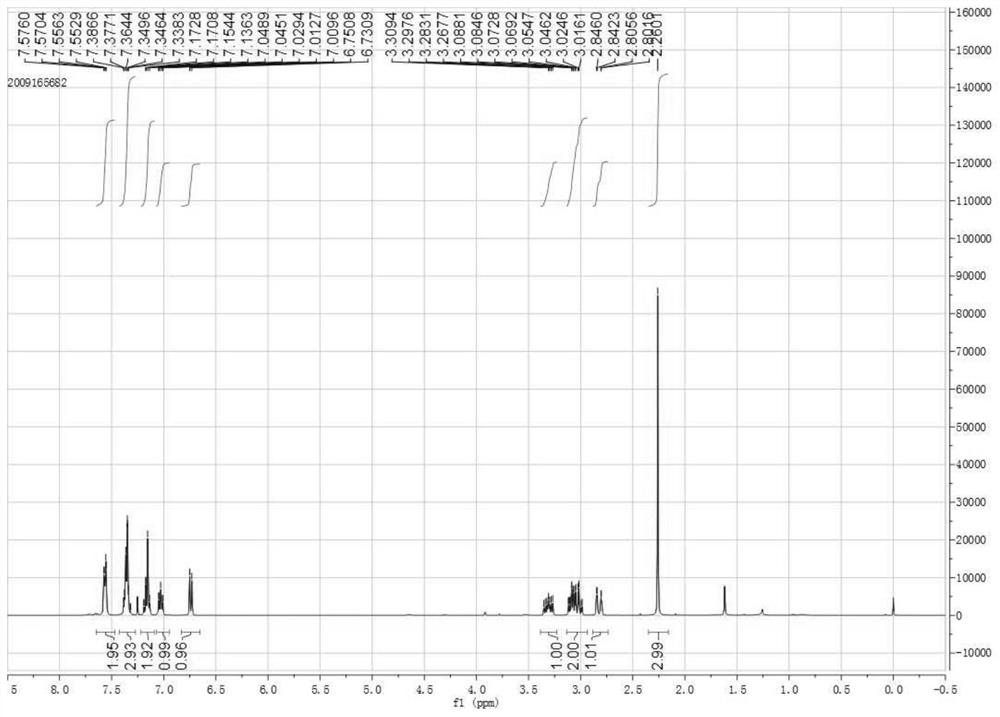
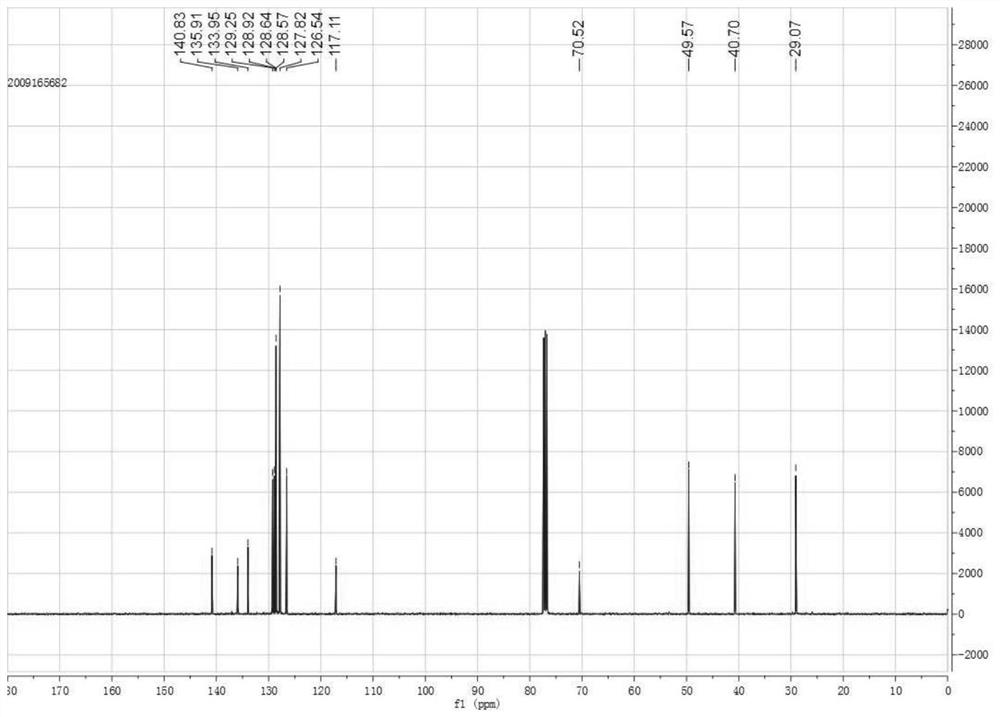
[0034] This example provides a synthetic method of 1-phenyl-1-cyano-2-methyl-1,2,3,4-tetrahydroisoquinoline, which is to weigh 103.7 mg of 1-phenyl- Add 3,4-dihydroisoquinoline and 212.9mg iodomethane into 3mL 1,2-dichloroethane, continue to stir and dissolve, heat to 40°C, maintain 40°C for 30min, continue to stir and cool down to room temperature, then Add 99.2 mg of trimethylsilyl cyanide, 58.1 mg of potassium fluoride and 26.5 mg of sodium carbonate, and continue to stir at room temperature for addition reaction for 24 hours. After the completion of the reaction, the reaction solution is extracted with dichloromethane and purified by column chromatography. , that is, 113.7mg of 1-phenyl-1-cyano-2-methyl-1,2,3,4-tetrahydroisoquinoline was obtained, the nuclear magnetic pattern is shown in figure 1 and figure 2 , the yield is 92%, and the chemical reaction formula is
[0035...
Embodiment 2~11
[0037] Embodiment 2~11 The synthetic method of 1-phenyl-1-cyano-2-methyl-1,2,3,4-tetrahydroisoquinoline
[0038] Examples 2 to 11 provide a synthetic method of 1-phenyl-1-cyano-2-methyl-1,2,3,4-tetrahydroisoquinoline respectively, and the synthetic methods are all the same as in the examples 1 are basically the same, the only difference is that the raw materials and some process parameters are different, and the specific data are shown in Table 1.
[0039] Table 1: Table of process parameters for the synthesis method of 1-phenyl-1-cyano-2-methyl-1,2,3,4-tetrahydroisoquinoline provided in Examples 2-11
[0040]
[0041] Other steps are all the same as in Example 1, and the obtained products are all 1-phenyl-1-cyano-2-methyl-1,2,3,4-tetrahydroisoquinoline, and the structural formula is
Embodiment 12
[0042] Example 12 A synthetic method of 1-cyano-2-methyl-1-(3-methylphenyl)-1,2,3,4-tetrahydroisoquinoline
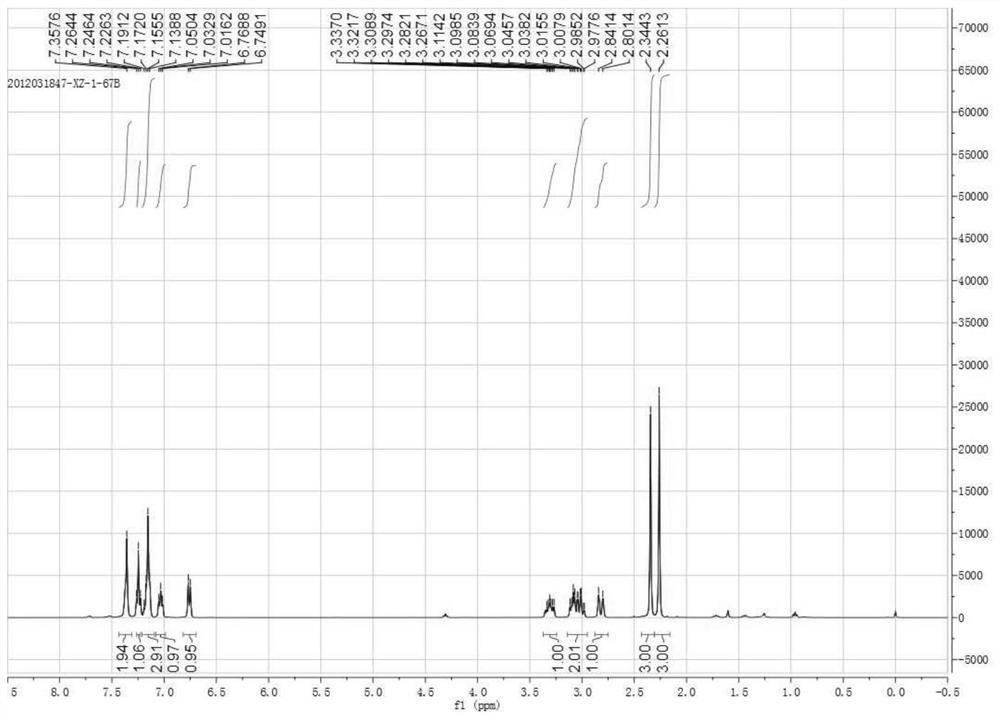
[0043] This embodiment provides a synthetic method of 1-cyano-2-methyl-1-(3-tolyl)-1,2,3,4-tetrahydroisoquinoline, which is to weigh 110.6mg 1 -(3-Tolyl)-3,4-dihydroisoquinoline, 212.9mg methyl iodide, added to 3mL 1,2-dichloroethane, stirring continuously to dissolve, heating to 40°C, maintaining 40°C for 30min , continue to stir and lower to room temperature, then add 99.2mg trimethylsilyl cyanide, 58.1mg potassium fluoride and 26.5mg sodium carbonate, continue to stir at room temperature for addition reaction for 48h, use TLC to detect the completion of the reaction, use dichloromethane The reaction solution was extracted and purified by column chromatography to obtain 130.3 mg of 1-cyano-2-methyl-1-(3-methylphenyl)-1,2,3,4-tetrahydroisoquinoline, yield 99%, the chemical reaction formula is
[0044]
[0045] 2-Methyl-1-(m-tolyl)-1,2,3,4-tetrahydroisoquinoline-1-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com