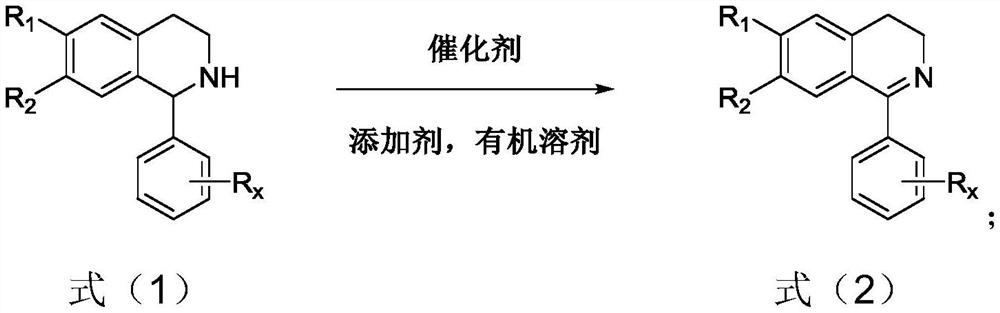
Method for synthesizing dihydroisoquinoline by catalyzing selective dehydrogenation of tetrahydroisoquinoline
A technology of tetrahydroisoquinoline and dihydroisoquinoline, applied in the direction of organic chemistry, can solve the problems of narrow substrate application range, unfavorable industrial production, harsh reaction conditions, etc., and achieve convenient and practical reaction operation and easy product , Increase the effect of reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Synthesis of 1-phenyl-3,4-dihydrooquinoline
[0038] This example provides a synthetic method of 1-phenyl-3,4-dihydrooquinoline, which is called 1-phenyl-1,2,3,4-tetrahydroisoquinoline (104.6 mg), respectively. ), Iodide (9.5 mg), 4-dimethylaminopyridine (12.2 mg), added to 3 mL of acetonitrile, continuously stirred, heated to 60 ° C, reacted for 10 h, and using TLC detection, after completion EtOAc.
[0039]
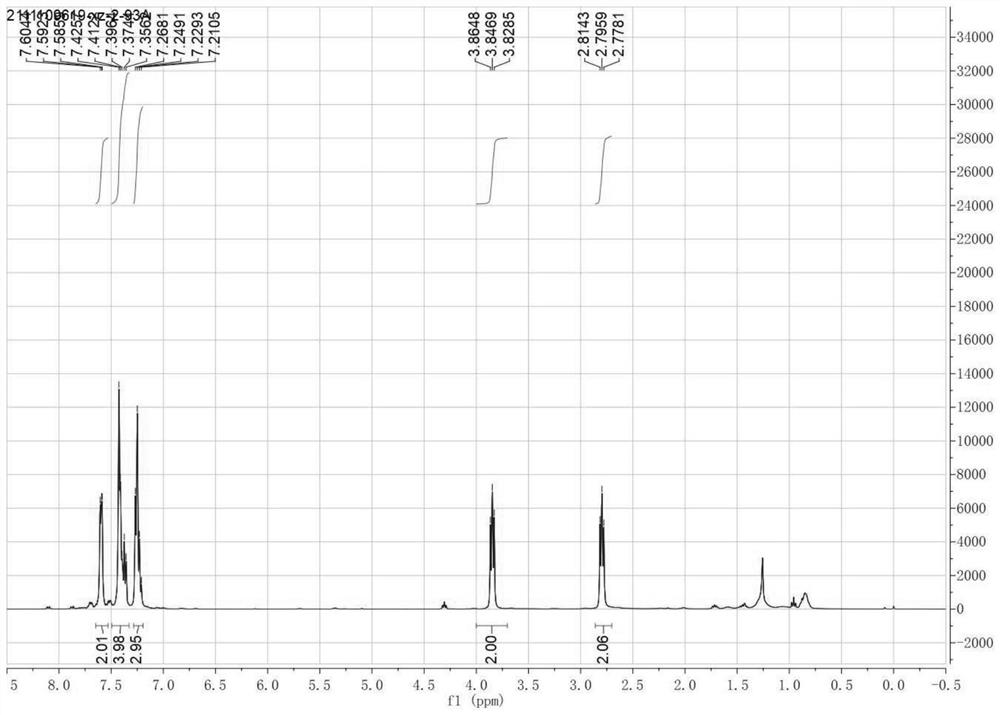
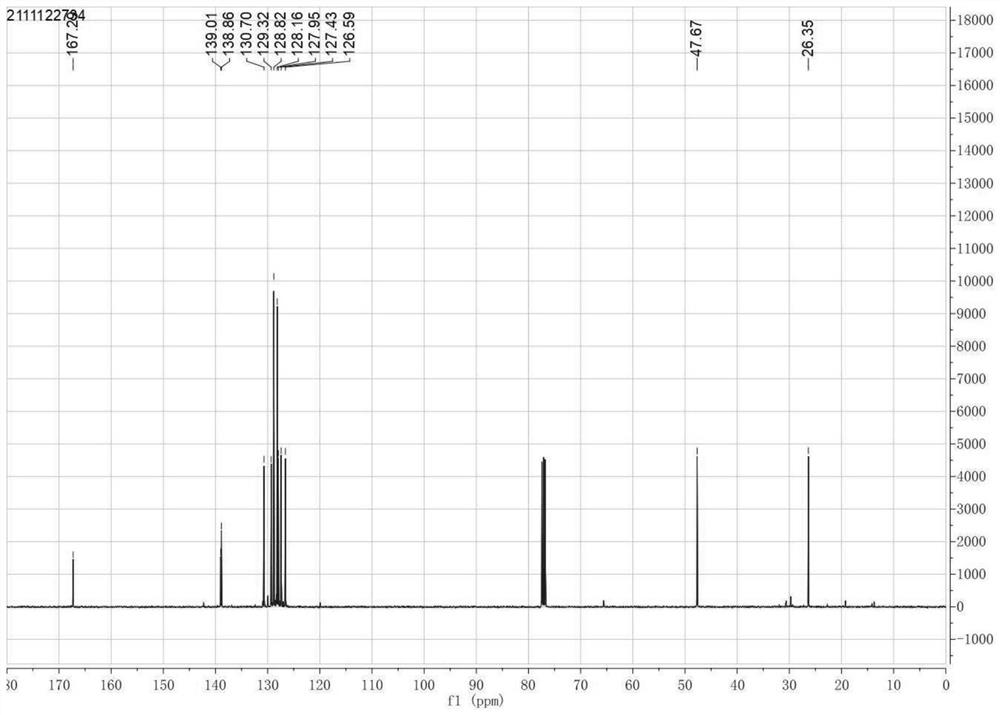
[0040] 1-phenyl-3,4-dihydrooquinoline (1-Phenyl-3, 4-DihydroisoisoisoisoisoisoisoisoisoisoisoisoisoisoisoisoisoiSoisoisoisoisoisoisoiSoisoisoiSoisoisoiSoisoisoiSoiSoiSoiSoiSoisoiSoiSoiSoisoiSoiSoiSoiSoiSoiSoQuinoline): Pale Yellowoil, 95% yield.r f = 0.45 (Petroleum ether / ethyl acetate 2: 1). 1 H NMR (400MHz, CDCL 3 Δ7.65-7.53 (m, 2H), 7.49-7.33 (m, 4H), 7.24 (m, 3H), 4.00-3.70 (m, 2H), 2.86-2.70 (m, 2H). 13 C NMR (100MHz, CDCL 3 Δ167.3, 139.0, 138.9, 130.7, 129.3, 128.8, 128.2, 128.0, 127.4, 126.6, 47.7, 26.4. Hydrogen core magnetic spectrum figure 1 A...
Embodiment 2~14
[0041] Example 2 to 14 Synthesis of 1-phenyl-3,4-dihydrooquinoline
[0042] The synthesis of 1-phenyl-3,4-dihydrooquinoline is provided, and the synthesis method is substantially the same as that of Example 1, and the difference is only different from the raw material and part of the process parameters. The specific data is shown in Table 1.
[0043] Table 1: Process Parameters
[0044]
[0045] Other steps were the same as in Example 1, the reaction was 1-phenyl-1,2,3,4-tetrahydroisoquinoline synthesized 1-phenyl-3,4-dihydrogen isoquine in a dehydrogenation reaction. Porroline, its reaction equation is
[0046]
[0047] As can be seen from Examples 1-14, the catalytic effect of iodide and bromide copper copper is better than that of other catalysts, and the use of acetonitrile is better than other solvents, using additives 4-dimethyl Aminopyridine can greatly increase the yield of the target product.
Embodiment 15
[0048] Example 15 Synthesis of 1- (2-methylphenyl) -3,4-dihydroisoquinoline
[0049] This example provides a synthesis method of 1- (2-methylphenyl) -3, 4-dihydroisoquinoline, which is respectively weighing 1-phenyl-1, 2, 3, 4-tetrahydrogen Isoquinoline (111.7 mg), copper iodide (9.5 mg), 4-dimethylaminopyridine (12.2 mg), added to 3 ml of acetonitrile, continuously stirred, heated to 60 ° C, maintaining 60 ° C for 24 h, utilization After the TLC was completed, purified from the column chromatography, i.e., 103.3 mg of 1-phenyl-3,4-dihydroisoquinoline, yield of 93%, chemical reaction formula
[0050]
[0051] 1- (2-methylphenyl) -3,4-dihydroisoquinoline (1- (O-Tolyl) -3, 4-dihydroisoisoSoQuinoline): Pale Yellow Solid, 93% Yield.R f = 0.34 (Petroleum ether / ethyl acetate 2: 1). 1 H NMR (400MHz, CDCL 3 ) δ7.37-7.20 (m, 6H), 7.16 (t, j = 7.5 Hz, 1H), 6.91 (D, J = 7.6Hz, 1H), 3.91 (m, 2H), 2.85 (T, J = 7.4 Hz, 2H), 2.13 (S, 3H). 13 C NMR (100MHz, CDCL 3 δ168.2, 139.0, 137.4, 135.8,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com