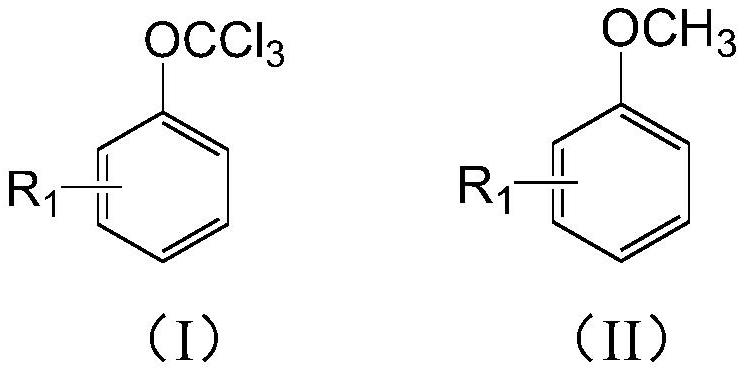
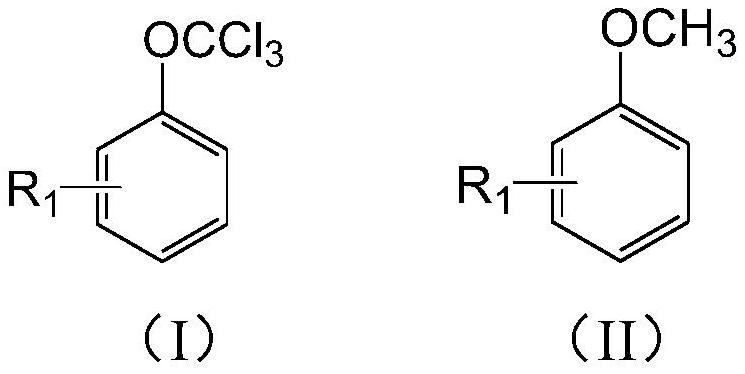
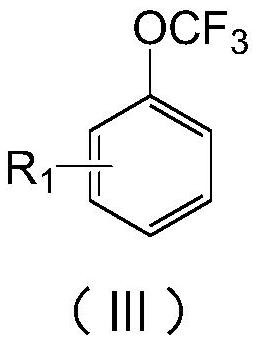
Preparation method of (trifluoromethoxy)benzene compound
A technology of trifluoromethoxybenzene and trichloromethoxybenzene, applied in the field of organic chemical industry synthesis, can solve problems such as solvent consumption, solvent cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] With 450g of fluorinated material rectification raffinate (main components: 4-chloro-trifluoromethoxybenzene 35.1%, difluoro-chloromethoxybenzene 20.4%, 4-chloro-difluoro-chloromethoxybenzene 15.7% %), 2.5g of phosphorus trichloride were added into a 1000mL reaction flask equipped with stirring, reflux condenser and chlorine pipe, and the temperature was raised to 120-130°C. Add 150g of anisole and 2.5g of AIBN, and at the same time feed chlorine gas to control the flow rate of chlorine gas to 40-50L / h. The reaction tail gas absorbs HCl therein through water, and then absorbs unreacted chlorine gas with 30% NaOH. After the anisole has been added, continue to feed chlorine, and add a mixed solution of 100g of fluorinated material rectification raffinate and 2g of AIBN. To the content of dichloromethoxybenzene in the reaction solution 2 Purging, precipitation, until the temperature of the kettle is 150°C / -0.096Mpa, the extracted solvent can continue to be used for the ch...
Embodiment 2
[0065] With 450g of fluorinated material rectification raffinate (main components: 20.2% of 4-chloro-trifluoromethoxybenzene, 35.8% of difluorochloromethoxybenzene, 16.7% of 4-chloro-difluorochloromethoxybenzene %), 2.5g of phosphorus trichloride were added into a 1000mL reaction flask equipped with stirring, reflux condenser and chlorine pipe, and the temperature was raised to 120-130°C. Add 150g of anisole and 2.5g of AIBN, and at the same time feed chlorine gas to control the flow rate of chlorine gas to 40-50L / h. The reaction tail gas absorbs HCl therein through water, and then absorbs unreacted chlorine gas with 30% NaOH. After the anisole has been added, continue to feed chlorine, and add a mixed solution of 100g of fluorinated material rectification raffinate and 2g of AIBN. To the content of dichloromethoxybenzene in the reaction solution 2 Purging, precipitation, until the temperature of the kettle is 150°C / -0.096Mpa, the extracted solvent can continue to be used for...
Embodiment 3
[0067] Add 600g of rectification raffinate of fluorinated materials (main components: 41% of 4-chloro-trifluoromethoxybenzene, 35% of 4-chloro-difluoro-chloromethoxybenzene) and 2.5g of phosphorus trichloride to 1000mL In a reaction flask equipped with stirring, a reflux condenser, and a chlorine pipe, the temperature is raised to 120-130°C. Add 200g of 4-chloroanisole and 2.5g of AIBN, and at the same time feed chlorine gas to control the flow rate of chlorine gas to 40-50L / h. The reaction tail gas absorbs HCl therein through water, and then absorbs unreacted chlorine gas with 30% NaOH. After adding 4-chloroanisole, continue to pass chlorine, and add the mixed solution of 100g rectification raffinate of fluorinated material and 2g AIBN. To the content of 4-chloro-dichloromethoxybenzene in the reaction solution 2 Purging, precipitation, until the temperature of the kettle is 150°C / -0.096Mpa, the extracted solvent can continue to be used for the chlorination reaction of 4-chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com