Protein compound, and preparation method and application thereof
A complex and protein technology, applied in the field of molecular biology, can solve the problems of high price and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] The preparation of embodiment 1 recombinant protein
[0129] In this example, the inventors designed the following 10 recombinant protein sequences based on (VPGKG) repeated polypeptide sequence and human interleukin 1 receptor antagonist (IL-1RA) sequence. For specific sequence information, see Table 2:
[0130] Table 2 Recombinant protein sequence characteristics
[0131]
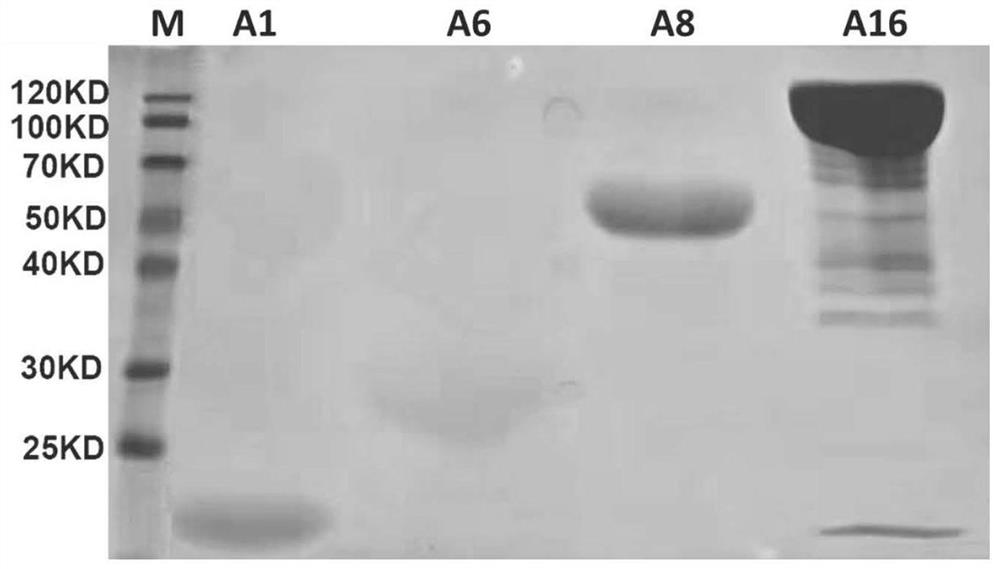
[0132] 1.1 Obtaining strains expressing recombinant proteins
[0133] The nucleotide sequences corresponding to the above 10 recombinant proteins were synthesized by Sangon Biotech (Shanghai) Co., Ltd. and constructed on the pET25b expression plasmid vector for transformation into prokaryotic expression competent BLR (DE3) of Escherichia coli. The specific embodiment is as follows: add 100 ng of pET25b-A1 (or A2~A10) plasmid into 100 μL of BLR Escherichia coli competent (purchased from Novagen) in an ice bath environment, and maintain it in an ice bath environment for 30 min; then The mixture ...
Embodiment 2
[0138] The preparation of embodiment 2 protein complexes
[0139] Protein complex 1: The purified recombinant protein A1 was mixed with carboxylated PEG at a molar ratio of 1:18, and stirred at 4°C for 1 hour. The mixture was loaded on a desalting chromatography column (purchased from GE), and eluted with 50 mM PB buffer with a pH of 7.2-7.4 to collect protein complex components.
[0140] Protein complex 2: The purified recombinant protein A2 was mixed with carboxylated PEG at a molar ratio of 1:72, and stirred at 4°C for 1 hour. The mixture was loaded on a desalting chromatography column (purchased from GE), and eluted with 50 mM PB buffer with a pH of 7.2-7.4 to collect protein complex components.
[0141] Protein complex 3: The purified recombinant protein A3 was mixed with carboxylated PEG at a molar ratio of 1:288, and stirred at 20-25°C for 4 hours. The mixture was loaded on a desalting chromatography column (purchased from GE), and eluted with 50 mM PB buffer with a p...
Embodiment 3
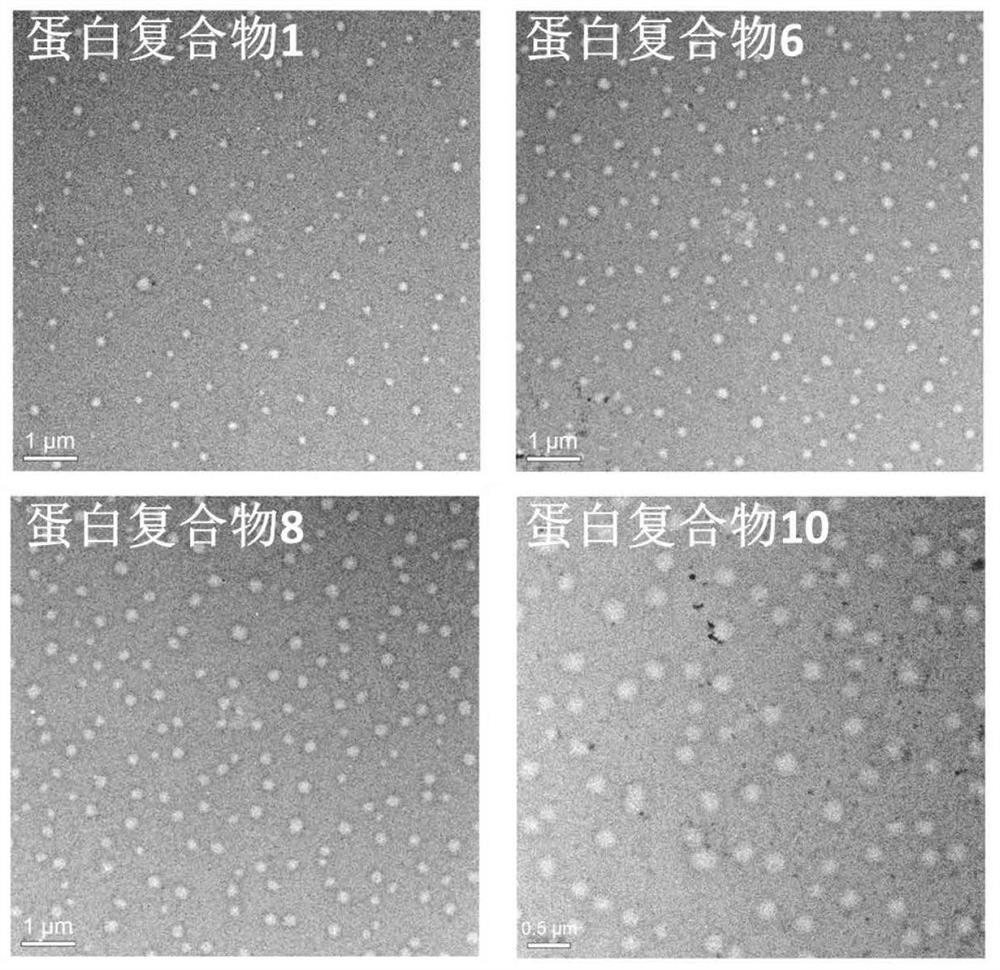
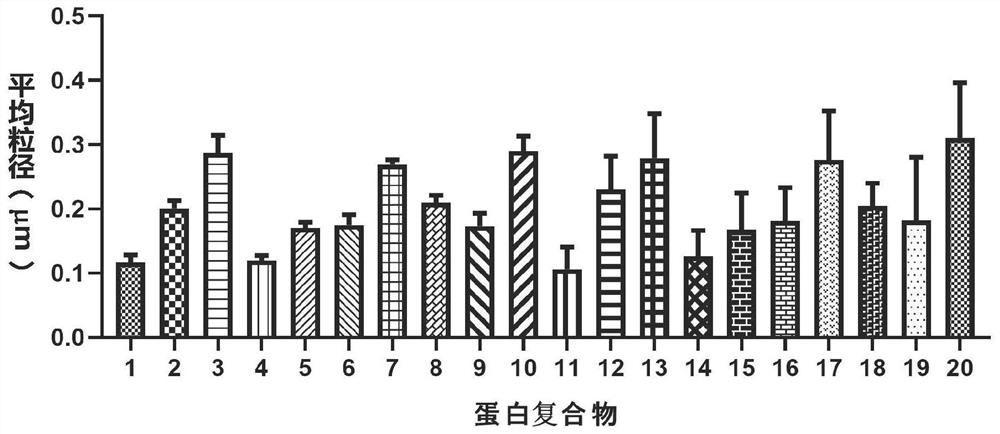
[0159] Example 3: Determination of protein complex particle size
[0160] The protein complex purified in Example 2 was detected by transmission electron microscopy, and the average particle size of the protein was analyzed. see results Figure 2~3 , figure 2 Indicates electron microscope pictures of protein complexes. image 3 Indicates the average particle size analysis results of proteins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com