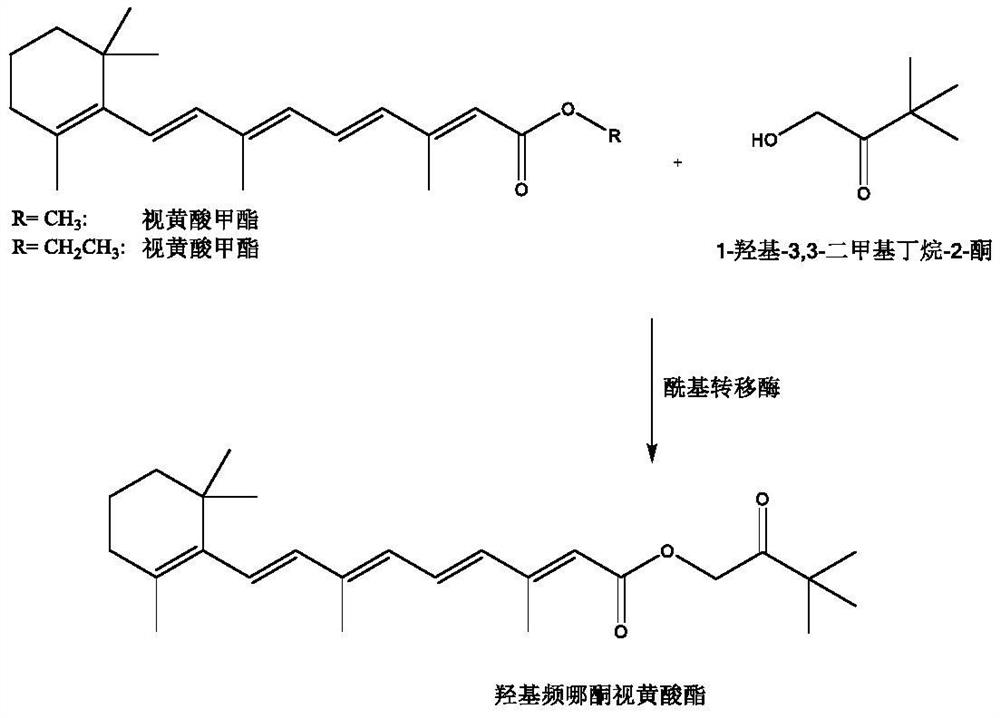
Method for synthesizing hydroxypinacolone retinoate (HPR) under catalysis of biological enzyme
A synthetic method, the technology of retinoic acid ester, applied in the field of synthesis of hydroxypinacolone retinoic acid ester, can solve the problems such as not very effective industrial operation, and achieve good substrate stability, high enzyme conversion rate, and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 500mL three-necked flask, add 200mL of 0.1M pH 8.0 PBS buffer solution, 0.2g of acyltransferase MsAcT, 10g of methyl retinoate, 1-hydroxy-3,3-dimethylbutan-2-one 4 g was reacted for 24 h at 25° C., stirred by a 200 rpm stirring paddle, and purged with 0.01 MPa nitrogen, and the conversion rate was 95% as determined by HPLC. Add hydrochloric acid to adjust the pH to 2-3, filter with celite, add an equal volume of ethyl acetate to extract twice, and rotary evaporate to obtain 10.2 g of the product with a purity of 98%.
Embodiment 2
[0018] In a 500mL three-neck flask, add 200mL of 0.1M pH 8.5 PBS buffer solution, 0.2g of acyltransferase MsAcT, 10g of methyl retinoate, 1-hydroxy-3,3-dimethylbutan-2-one 5 g was reacted for 24 h at 30° C., stirred by a stirring paddle at 200 rpm, and purged with nitrogen gas at 0.01 MPa, and the conversion rate was 95% as determined by HPLC. Add hydrochloric acid to adjust the pH to 2-3, filter with diatomaceous earth, add an equal volume of ethyl acetate to extract twice, and rotary evaporate to obtain 11.2 g of the product with a purity of 98%.
Embodiment 3
[0020] In a 500mL three-neck flask, add 200mL of 0.1M pH 9 PBS buffer solution, 0.2g of acyltransferase MsAcT, 10g of methyl retinoate, 1-hydroxy-3,3-dimethylbutan-2-one 6 g, at 30° C., stirring with a 200 rpm stirring paddle, and purging with 0.01 MPa nitrogen, reacted for 24 hours, and the conversion rate was 95% as detected by HPLC. Add hydrochloric acid to adjust the pH to 2-3, filter with diatomaceous earth, add an equal volume of ethyl acetate to extract twice, and rotary evaporate to obtain 11.1 g of the product with a purity of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com