Synthesis method of prucalopride impurities
A technology of prucalopride and synthetic method, which is applied in the field of organic drug synthesis, can solve the problems of unobserved degradation, unobserved full degradation, etc., and achieve the effects of short reaction time, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
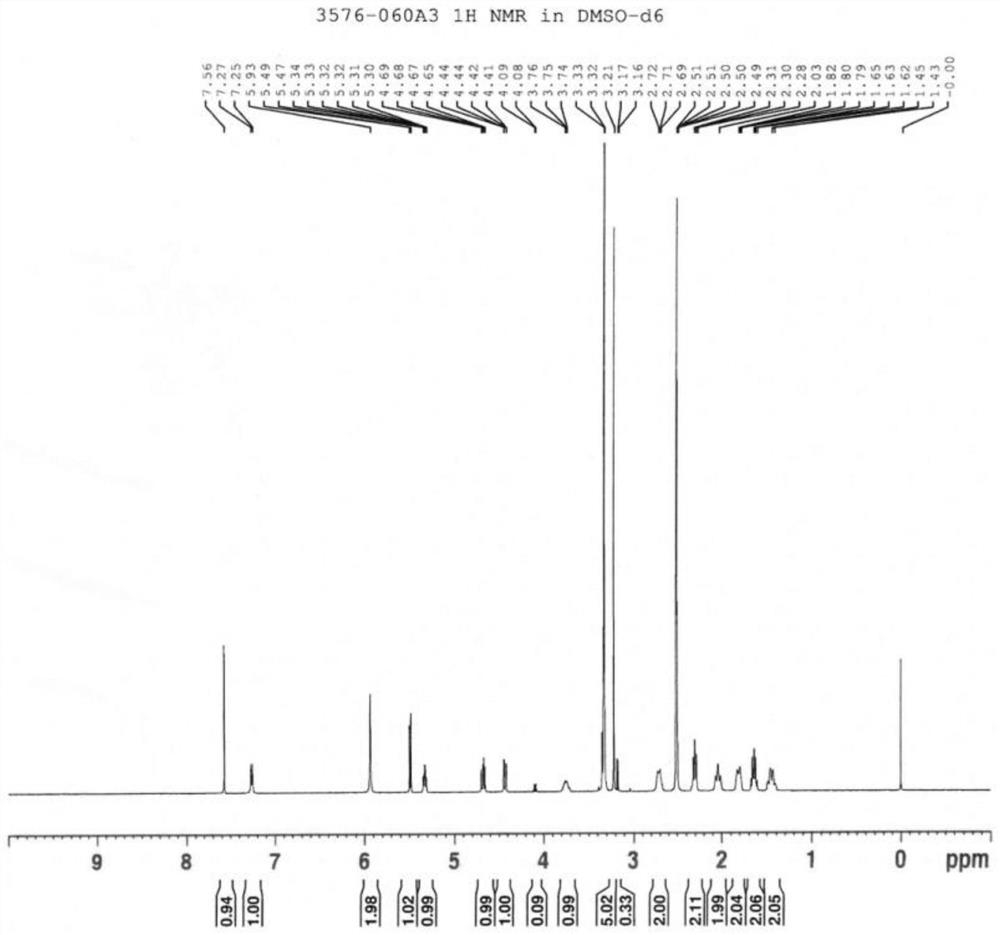
[0031] Add 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofuran carboxamide ( 74.7mg, 0.20mmol), Cu 2 O (1.4mg, 0.01mmol), 4-dimethylaminopyridine (24.4mg, 0.20mmol), N-hydroxyphthalimide (6.6mg, 0.04mmol), and acetonitrile (2.0ml) were stirred and dissolved. The reaction mixture was washed with O 2 Bubble for 30min and seal quickly. Then the reaction solution was stirred at 120° C. for 12 h. The reaction mixture was concentrated to dryness under reduced pressure. The residue was purified by column chromatography to give 4-amino-5-chloro-N-(1-(3-methoxypropyl)piperidin-4-yl)benzofuran-7-carboxamide (38.8 mg, Yield 53%).
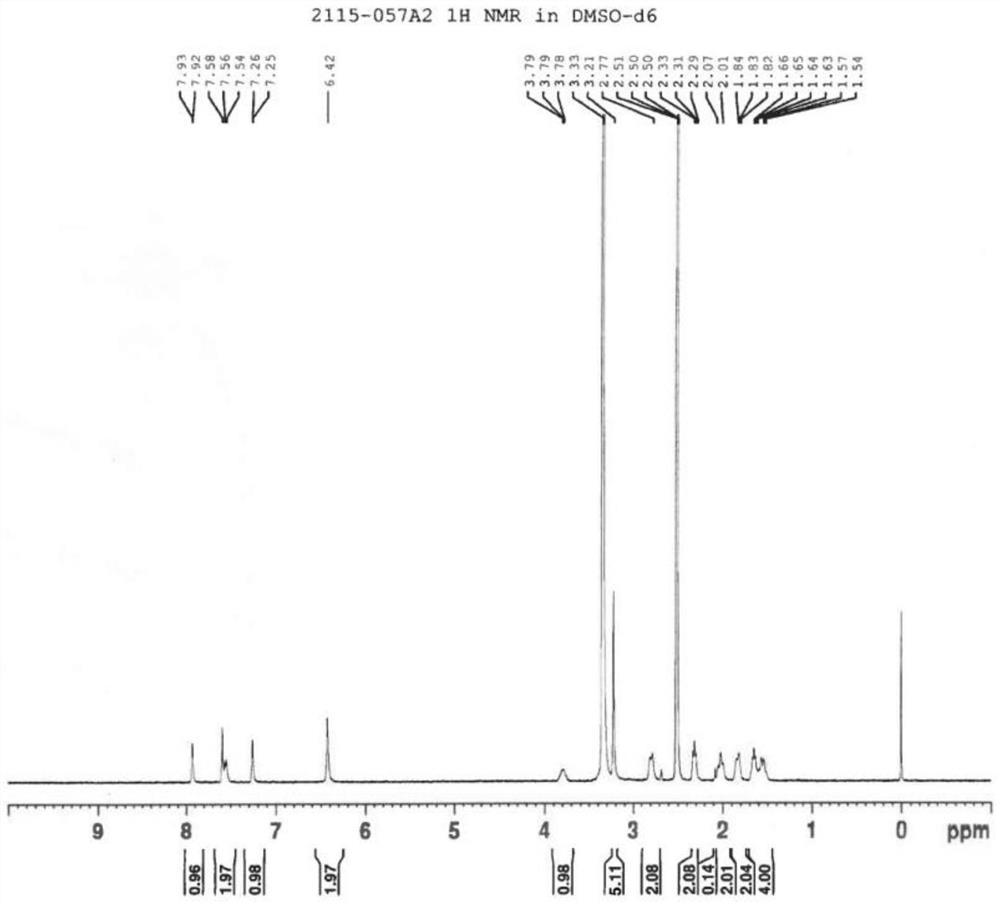
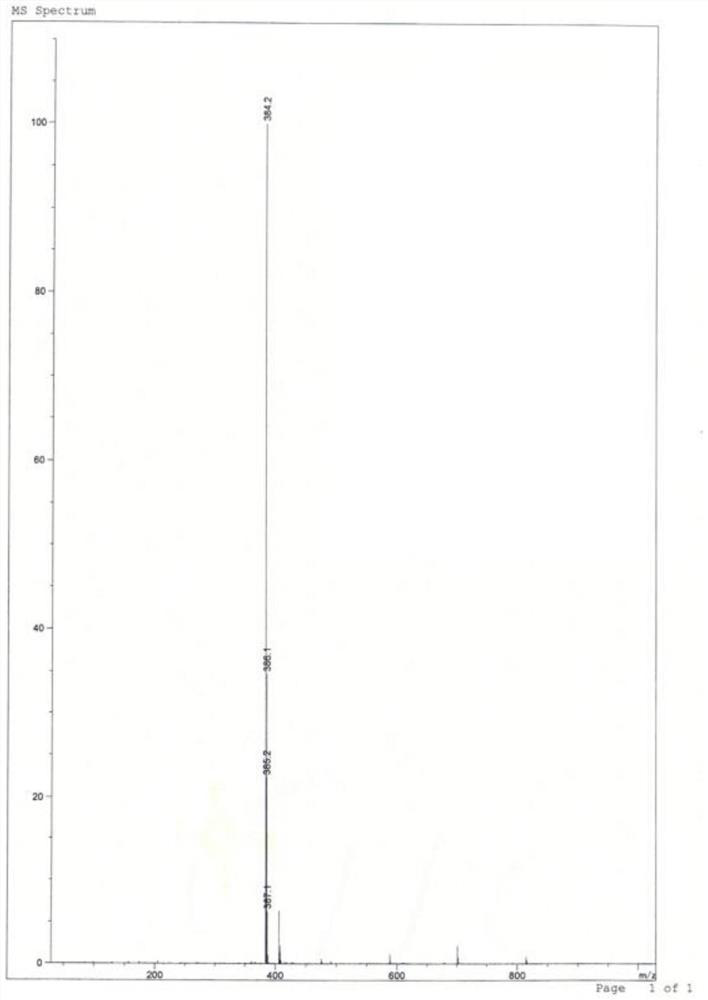
[0032] LC-MS (m / z): 364.1 [M-H] - ; 1 H-NMR (400MHz, DMSO-d6) δ: 7.92(d, J=0.4Hz=CH), 7.58(2H, CONH and ArH), 7.25(d, J=0.4Hz,=CH), 6.42(1H, s,-NH 2 ),3.78(1H,m,-CH),3.21(5H,m,-OCH 2and OCH 3 ),2.77(2H,m,-CH 2 in Piperidine),2.30(2H,m,-CH 2 ),2.01(2H,m,-CH 2 in Piperidine), 1.83 (2H, m, -CH 2 in Piperidine), 1.65 (...
Embodiment 2
[0034] Under air, add 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzo Furocarboxamide (74.7mg, 0.20mmol), N-hydroxyphthalimide (6.6mg, 0.04mmol) and acetonitrile (3.0mL) were stirred and dissolved. The reaction vial was sealed and the reaction mixture was stirred at 120 °C for 16 hours. The reaction mixture was concentrated to dryness under reduced pressure, and the residue was purified by column chromatography to obtain 4-amino-5-chloro-N-(1-(3-methoxypropyl)piperidin-4-yl)benzofuran -7-Carboxamide (40.2 mg, yield 55%).
Embodiment 3
[0036] Under air, add 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzo Furocarboxamide (74.7mg, 0.20mmol), N-hydroxyphthalimide (6.6mg, 0.04mmol) and Cu(OAc) 2 ·H 2 O (2.0 mg, 0.01 mmol), the reaction vial was sealed and the reaction mixture was stirred at 120 °C for 16 hours. The reaction mixture was concentrated to dryness under reduced pressure, and the residue was purified by column chromatography to obtain 4-amino-5-chloro-N-(1-(3-methoxypropyl)piperidin-4-yl)benzofuran -7-Carboxamide (49.8 mg, yield 68%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com