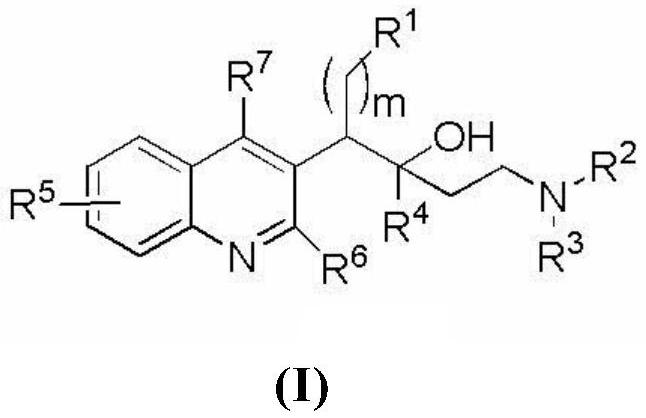
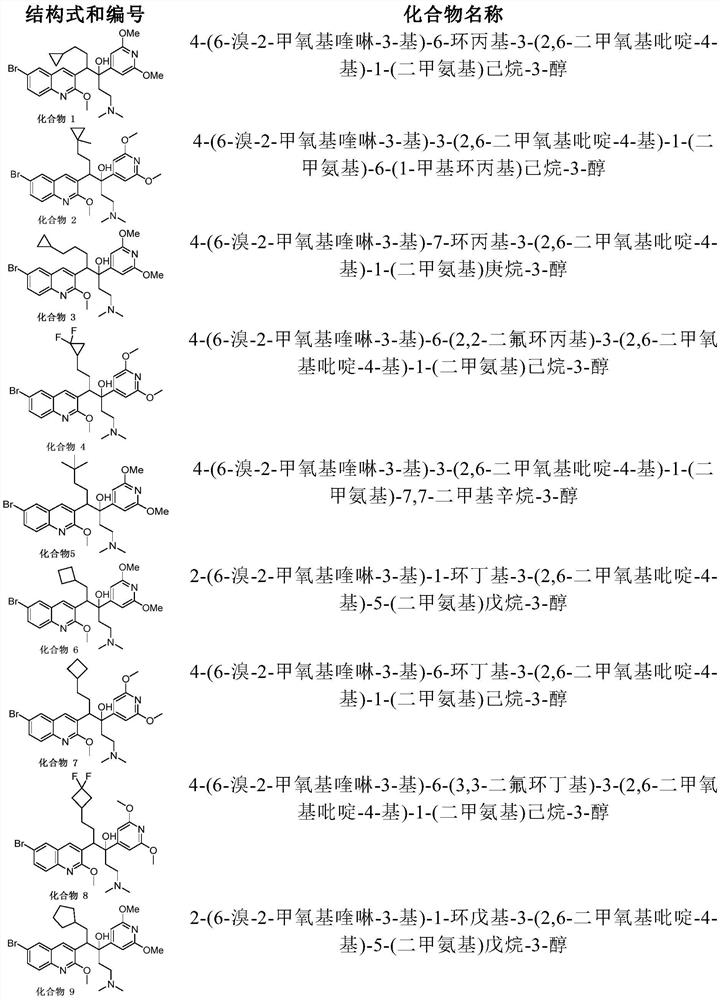
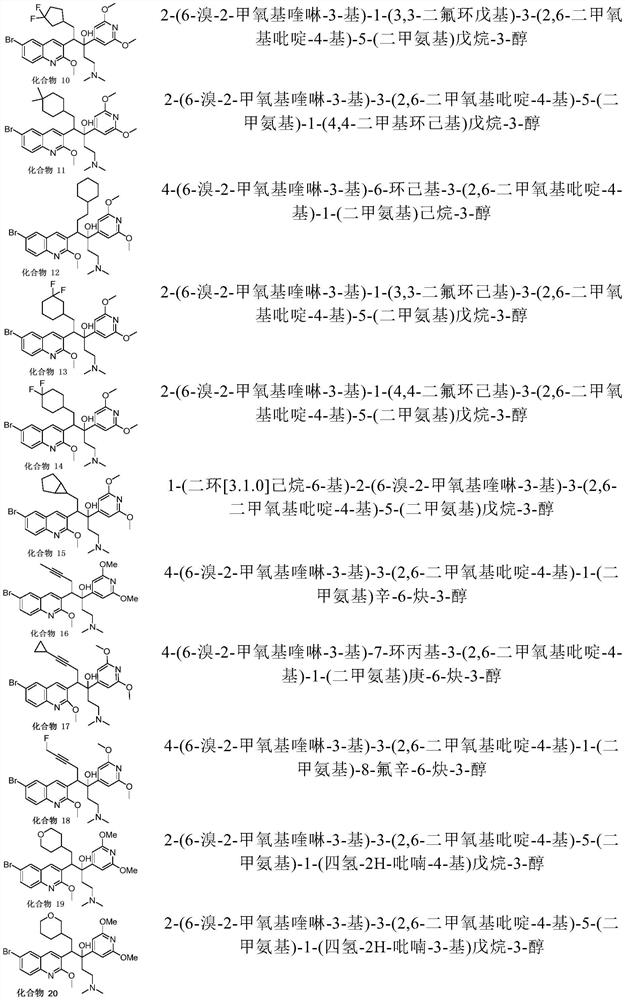
Quinoline compound, preparation method and application thereof
A compound, unsubstituted technology, applied in the fields of pharmacy, medicinal chemistry and pharmacology, which can solve problems such as unclear specific reasons, low bioavailability, safety risks, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0210] Embodiment 1: the preparation of compound 1
[0211]
[0212] Using intermediate IV-1 and 2-cyclopropyl ethyl methanesulfonate as starting materials, compound 1 was prepared according to the following reaction scheme
[0213]
[0214] Preparation of Compound 1-1
[0215] Intermediate IV-1 (834mg, 2.00mmol), 2-cyclopropyl ethyl methanesulfonate (966mg, 5.46mmol), K 2 CO 3 (829mg, 6.00mmol), KI (33mg, 0.20mmol) were added to acetone (10mL), protected by Ar gas, and reacted overnight at 50°C. TLC detected a small amount of remaining raw material, cooled to room temperature and directly mixed the sample through the column (petroleum ether: ethyl acetate = 40:1-30:1) to obtain a light yellow solid: 644 mg, yield 66%. LC-MS(ESI):485.1[M+H] +
[0216] Preparation of compound 1-2
[0217] CuBr·Me 2 S (27mg, 0.1mmol) was added to a 25mL three-neck flask, under the protection of Ar gas, a solution of newly prepared allyl zinc bromide in tetrahydrofuran (4mL, 3.98mmol...
Embodiment 2
[0234] Embodiment 2: the preparation of compound 2
[0235]
[0236] Using intermediate IV-1 and 2-(1-methylcyclopropyl)ethyl p-toluenesulfonate as starting materials, using the same reaction process as the preparation of compound 1, compounds 2A (104 mg) and 2B (85 mg ).
[0237] 2A (104mg), resolved by chiral HPLC to obtain 2A-1 (45mg) and 2A-2 (39mg)
[0238] LC-MS(ESI):572.2[M+H] +
[0239] 1 H NMR (400MHz, CDCl 3 )δ:8.17(s,1H),7.87(d,J=2.2Hz,1H),7.68(s,1H),7.64(d,J=2.2Hz,1H),6.57(s,2H),4.08( s,3H),3.96(s,6H),2.03(s,1H),1.96(s,6H),1.88(s,2H),1.86–1.76(m,2H),1.52–1.34(m,4H) ,1.26–1.22(m,3H),0.74(s,4H).
[0240] 2B (85mg), resolved by chiral HPLC to obtain 2B-1 (36mg) and 2B-2 (29mg)
[0241] LC-MS(ESI):572.3[M+H] +
[0242] 1 H NMR (400MHz, CDCl 3 )δ:8.03(s,1H),7.73(d,J=2.2Hz,1H),7.54(s,1H),7.50(d,J=2.2Hz,1H),6.43(s,2H),3.94( s,3H),3.82(s,6H),1.89(s,1H),1.82(s,6H),1.74(s,2H),1.72–1.62(m,2H),1.38–1.20(m,4H) ,1.12–1.08(m,3H),0.60(s,4H).
Embodiment 3
[0243] Embodiment 3: the preparation of compound 3
[0244]
[0245] Using intermediate IV-1 and 3-cyclopropylpropyl p-toluenesulfonate as starting materials, using the same reaction process as the preparation of compound 1, compounds 3A (69 mg) and 3B (87 mg) were obtained
[0246] 3A (69mg) was resolved by chiral HPLC to obtain 3A-1 (22mg) and 3A-2 (20mg)
[0247] LC-MS(ESI):572.2[M+H] +
[0248] 1 H NMR (400MHz, CDCl 3 )δ: 8.23(s, 1H), 7.90(d, J=2.2Hz, 1H), 7.72(d, J=8.8Hz, 1H), 7.65(dd, J=8.9, 2.2Hz, 1H), 6.56( s,2H),4.11(s,3H),3.97(s,6H),3.60(dd,J=12.0,3.0Hz,1H),2.21(ddd,J=26.4,9.4,5.4Hz,1H),1.94 (s,9H),1.51–1.40(m,2H),1.25(s,2H),0.96(t,J=5.5Hz,2H),0.43–0.31(m,1H),0.20(d,J=7.8 Hz,2H),0.14–0.28(m,2H).
[0249] 3B (87mg) was resolved by chiral HPLC to obtain 3B-1 (27mg) and 3B-2 (32mg)
[0250] LC-MS(ESI):572.2[M+H] +
[0251] 1 H NMR (400MHz, CDCl 3 )δ: 8.18(s, 1H), 7.85(d, J=2.2Hz, 1H), 7.67(d, J=8.8Hz, 1H), 7.60(dd, J=8.9, 2.2Hz, 1H), 6.51( s,2H),4.06(s,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com