Antibody against precursor brain-derived neurotrophic factor and application thereof
A technology of neurotrophic factors and antibodies, applied in application, anti-growth factor immunoglobulin, genetic engineering, etc., can solve the problem of lack of specific monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
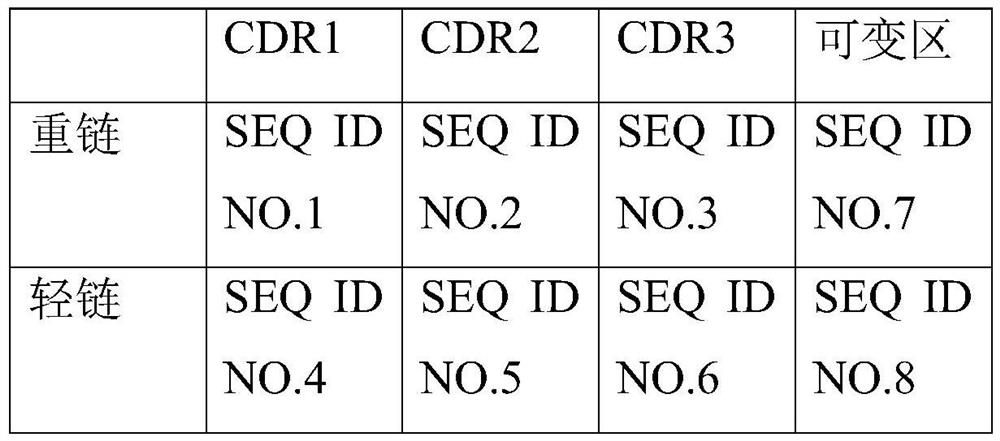
[0037] Anti-ProBDNF Antibody Screening
[0038] The amino acid sequence (SEQ ID NO.11) of the antigen ProBDNF used is as follows:
[0039] CDQKVRPNEENNKDA.
[0040] The polypeptide in the pro fragment of the above proBDNF protein is used as the antigen for immunization and the antigen for screening, and the proBDNF protein itself is also used as the antigen protein for screening. The immunized animals are female Balb / c mice aged 6-8 weeks, injected with antigen, and quickly immunized with a special adjuvant for about two weeks. After the animal was immunized, the antibody titer was detected. When the antibody titer was high enough, the animal was anesthetized with isoflurane and then sacrificed. The lymph node was taken, digested and fused with the myeloma. Use DMEM to culture sp2 / 0 myeloma cells. After the cells are in good condition and the rate of viable cells is over 80%, they are fused with lymphocytes through polyethylene glycol. Then use the HAT screening medium to c...
Embodiment 2
[0053] Purification of anti-ProBDNF antibody
[0054] The antibody gene sequence measured above was codon-optimized, synthesized and inserted into the pcDNA3.4 vector, and the Fc region used the original sequence of the antibody subtype IgG2b. The constructed expression vector was transferred into Escherichia coli for amplification and expression, and the expression plasmid was purified. The plasmid was electrotransfected into 293 cells. After 2 days of cultivation, the antibody protein in the supernatant was extracted, and the protein A affinity column was used for The antibody was purified and identified using HPLC-SEC and SDS-Page to determine its concentration and purity. After testing, the antibody concentration was 0.895 mg / ml, and the purity reached 94%.
Embodiment 3
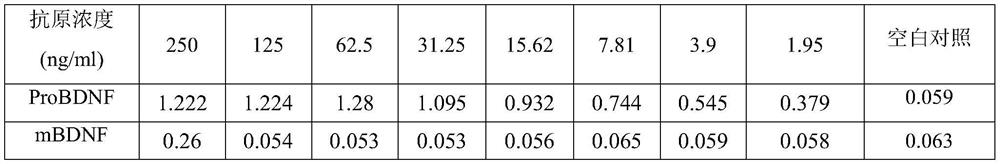
[0056] Specific detection of anti-mBDNF antibody
[0057] Antigen sample: mBDNF or ProBDNF (amino acid sequence shown in SEQ ID NO.11)
[0058] Detection by ELISA method:
[0059] 1) Coating: A 96-well plate was coated with 50 μl of mBDNF or proBDNF protein at a concentration of 2 μg / ml overnight at 4° C., then washed three times with PBST (Tween 0.05%), and patted dry.
[0060] 2) Blocking: Add 200 μl of the prepared blocking solution (PBST solution containing 1% BSA) to a 96-well plate, block at room temperature for 1 hour, wash the plate four times, and pat dry.
[0061] 3) Add 50 μl of sample (purified antibody provided in Example 2), incubate at room temperature for 1 hour, wash the plate four times, and pat dry.
[0062] 4) Secondary antibody incubation: Secondary antibody (1:2000) was prepared with blocking solution, 100 μl of secondary antibody was added to each well, incubated at room temperature for 1 hour, washed four times, and patted dry.
[0063] 5) 100 μl of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com