Non-natural amino acid short peptide and application thereof in tumor resistance
An unnatural amino acid, amino acid technology, applied in antitumor drugs, medical preparations containing active ingredients, peptides, etc., can solve the problems of inaccurate targeting of natural active peptides, normal cell toxicity, poor stability, etc. The effect of tumor cell proliferation and migration ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis and screening of small molecule short peptides
[0031] (1) Synthesis of unnatural amino acid short peptides
[0032] The present invention is based on the heptapeptide library established by our laboratory, and synthesizes non-natural amino acid short peptides through chemical synthesis. For the specific synthesis method, please refer to the Chinese patent application (CN111228508A-a multi-target anti-tumor polypeptide drug couple) Compound and its preparation method and application) Example 1:
[0033] Amide resin (RINK AMIDE RESIN; C 30 h 26 NO 5 R) Purchased from P3BioSystems in the United States (the degree of substitution is 0.503mmol / g); pretreatment before use: add DMF (N,N-dimethylformamide) to the amide resin, soak for 2-3 hours, and then discard Remove the solution to obtain the pretreated amide resin;
[0034] Fmoc-Lys(Dde)-OH, Fmoc-AEEA ([2-[2-(Fmoc-amino)ethoxy]ethoxy]acetic acid), Fmoc-Glu(OtBu)-OH, Fmoc-Lys(Boc) , Fmoc-AEEA-OH,...
Embodiment 2
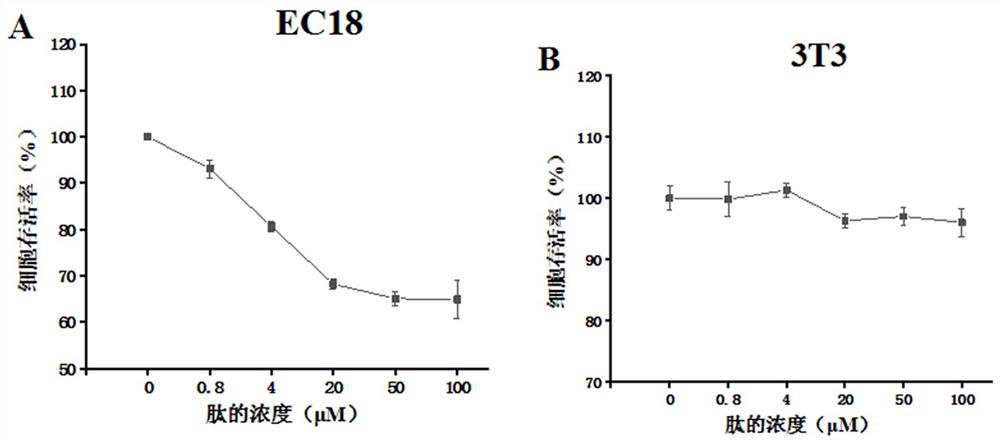
[0054] Example 2: Detection of broad-spectrum anti-tumor properties of short peptide CR1
[0055] (1) Select the test cell line
[0056] Alternative cell lines are A549 (human lung cancer cells), MHCC-97H (human highly metastatic liver cancer cells), MCF-7 (human breast cancer cells), DU145 (human prostate tumor cells), all of which were purchased from Shanghai cell bank.
[0057] (2) Test method
[0058] The test method is the same as step ② in Example 1, and the cell lines are A549, MHCC-97H, MCF-7, and DU145.
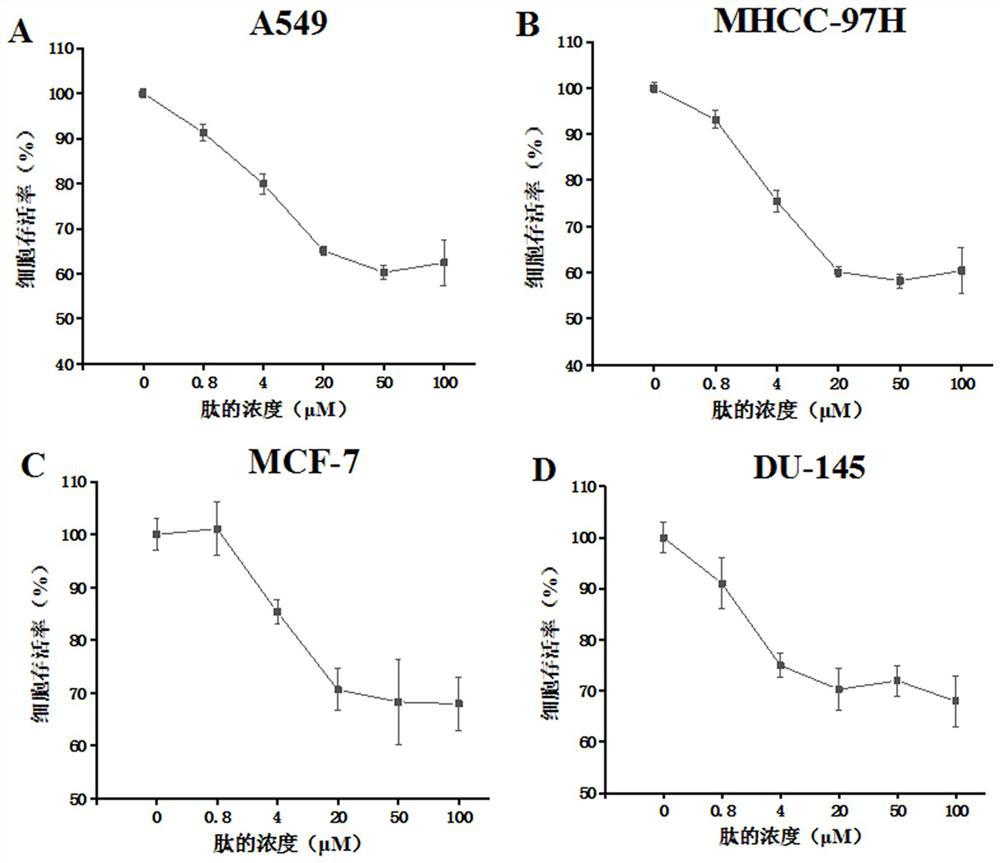
[0059] (3) Test results
[0060] The result is as figure 2 As shown: the CR1 short peptide has a certain level of inhibition on the proliferation of the above four cell lines, and all of them are concentration-gradient dependent. The maximum inhibitory efficiency can be obtained with a basic administration concentration of no more than 20 μM.
Embodiment 3
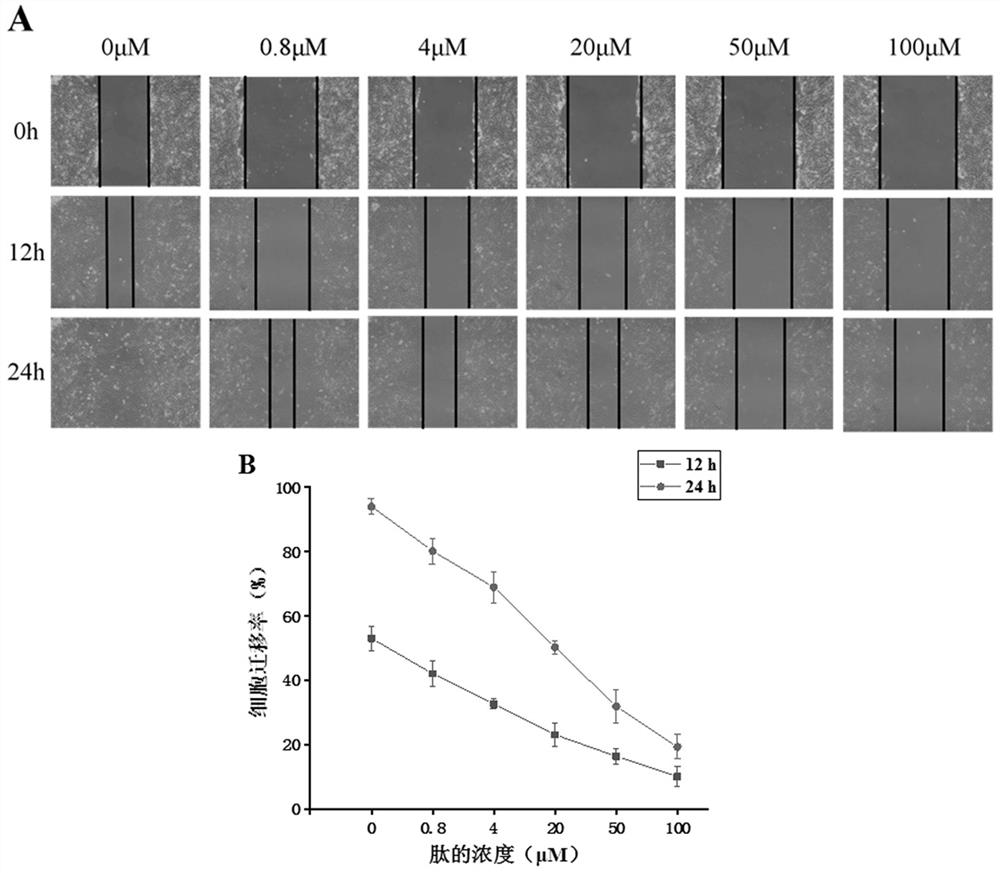
[0061] Example 3: Detection of CR1 short peptide inhibiting migration of MHCC-97H in vitro
[0062] (1) Select experimental cells
[0063] MHCC-97H cells themselves have good migration ability, and CR1 short peptide has a significant effect on the proliferation of MHCC-97H cells in the experiment of inhibiting cell proliferation, so MHCC-97H cells were selected as the experimental subjects in this experiment.
[0064] (2) Test method
[0065] A. Cultivate MHCC-97H cells in RPMI 1640 medium supplemented with 10% (v / v) fetal bovine serum to the logarithmic phase, digest and collect the cells, spread them evenly in a 12-well plate, and wait until the cells are confluent to 90% DMEM medium containing 0.5% (v / v) fetal bovine serum was starved for 24 hours, then scratched, and washed 3 times with PBS buffer, and then the medium was changed to add 0.5% (v / v) fetal bovine serum and CR1 short peptide in DMEM medium, and the scratches were photographed and recorded, and three replicat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com