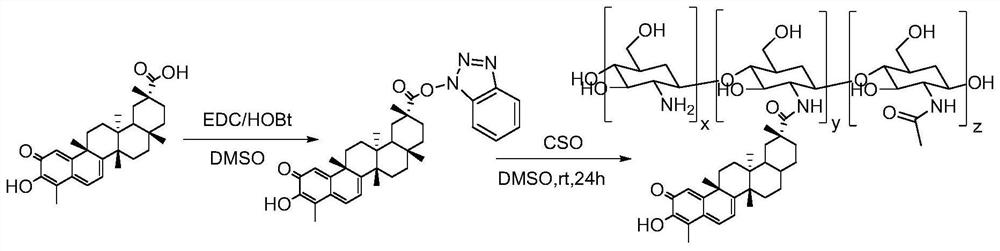
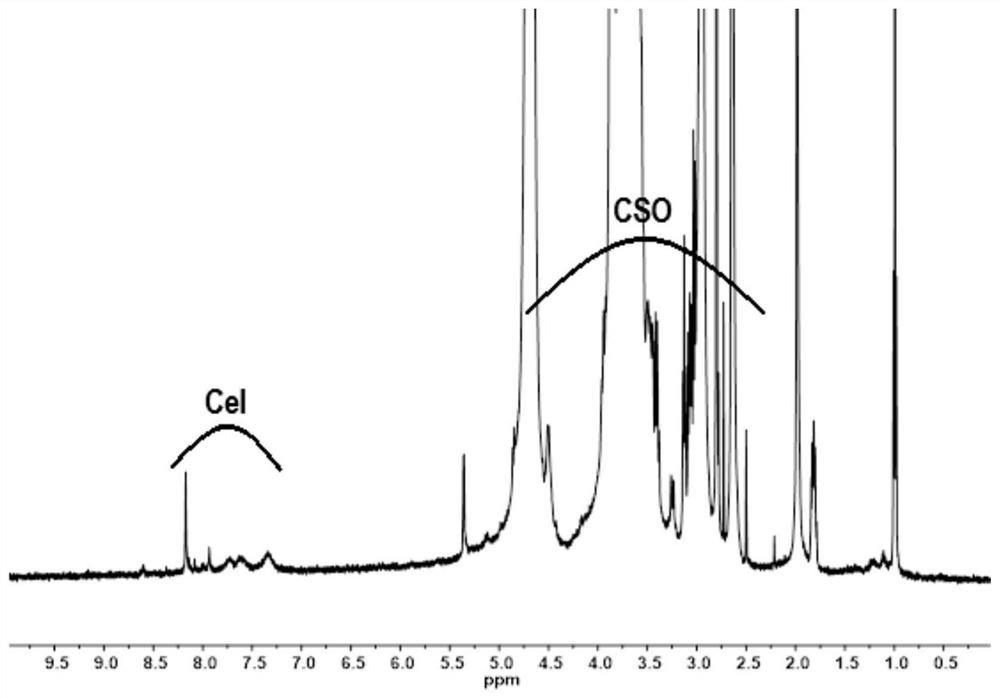
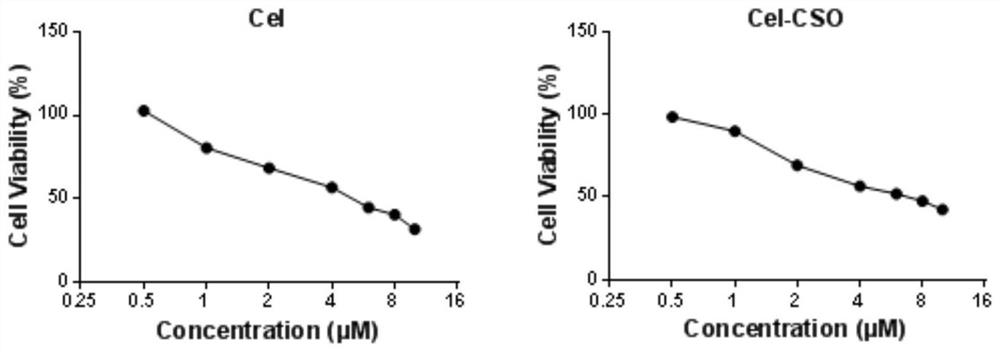
Preparation method and use of tripterine-chitosan oligosaccharide coupled drug
A technology of tripterine and chitosan oligosaccharides, which is applied in the field of medicine, can solve the problems of short biological half-life, poor water solubility, and large toxic and side effects of tripterine, and achieve weight loss, mild reaction conditions, and good water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthetic steps of tripterine-chitooligosaccharide conjugated drug one (CEL-CSO1) are:
[0026] 1) Activate tripterine
[0027] Weigh 100mg (0.22mmol) tripterine and dissolve in 3mL DMSO, then add 64mg 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC , 0.33mmol) and 45mg of 1-hydroxybenzotriazole (HOBt, 0.33mmol), stirred and activated at 25°C for 6h. 1), then the mobile phase was spin-dried to obtain 88.7mg solid product (71%);
[0028] 2) Synthesis of conjugated drug CEL-CSO1
[0029] Weigh 800 mg chitosan oligosaccharide (MW=1000) and dissolve it in dimethyl sulfoxide at 55°C, cool to 25°C after dissolving, add activated tripterine drop by drop, stir at 25°C for 24 hours, transfer the reaction solution into In a dialysis bag with a molecular weight cut-off of 500 Da, low-temperature dialysis was performed with double-distilled water for 24 hours, and the water was changed every 6 hours. The liquid in the bag was collected and freeze-dried to ob...
Embodiment 2
[0031] Tripterine-chitooligosaccharide conjugated drug two (CEL-CSO3) synthesis steps are:
[0032] 1) Activate tripterine
[0033] Weigh 100mg (0.22mmol) tripterine and dissolve in 3mL DMSO, then add 64mg 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC , 0.33mmol) and 45mg 1-hydroxybenzotriazole (HOBt, 0.33mmol), stirred and activated at 25°C for 6h, after the reaction was completed, the sample was wet-loaded and purified by silica gel chromatography (mobile phase CH2Cl2 / CH3OH, 30:1 ~20:1), then the mobile phase was spin-dried to give 88.7 mg of solid product (71%).
[0034] 2) Synthesis of coupled drug CEL-CSO3
[0035] Weigh 800 mg chitosan oligosaccharide (MW=3000) and dissolve it in dimethyl sulfoxide at 55°C, cool to 25°C after dissolving, add activated tripterine dropwise, stir at 25°C for 24h, and dissolve the reaction solution Transfer it into a dialysis bag with a molecular weight cut-off of 500 Da, dialyze with double distilled water at low tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com