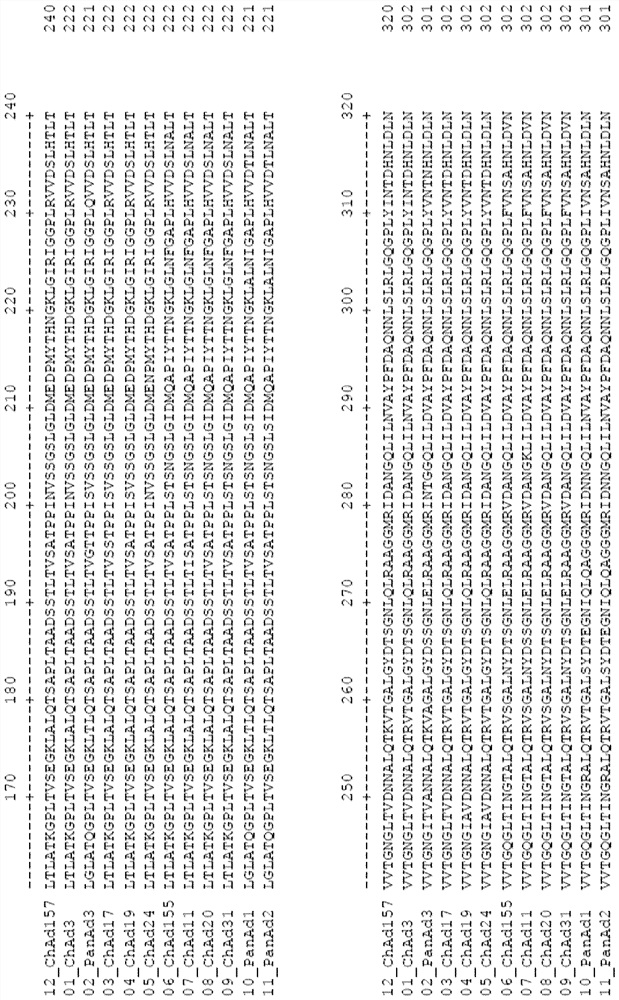
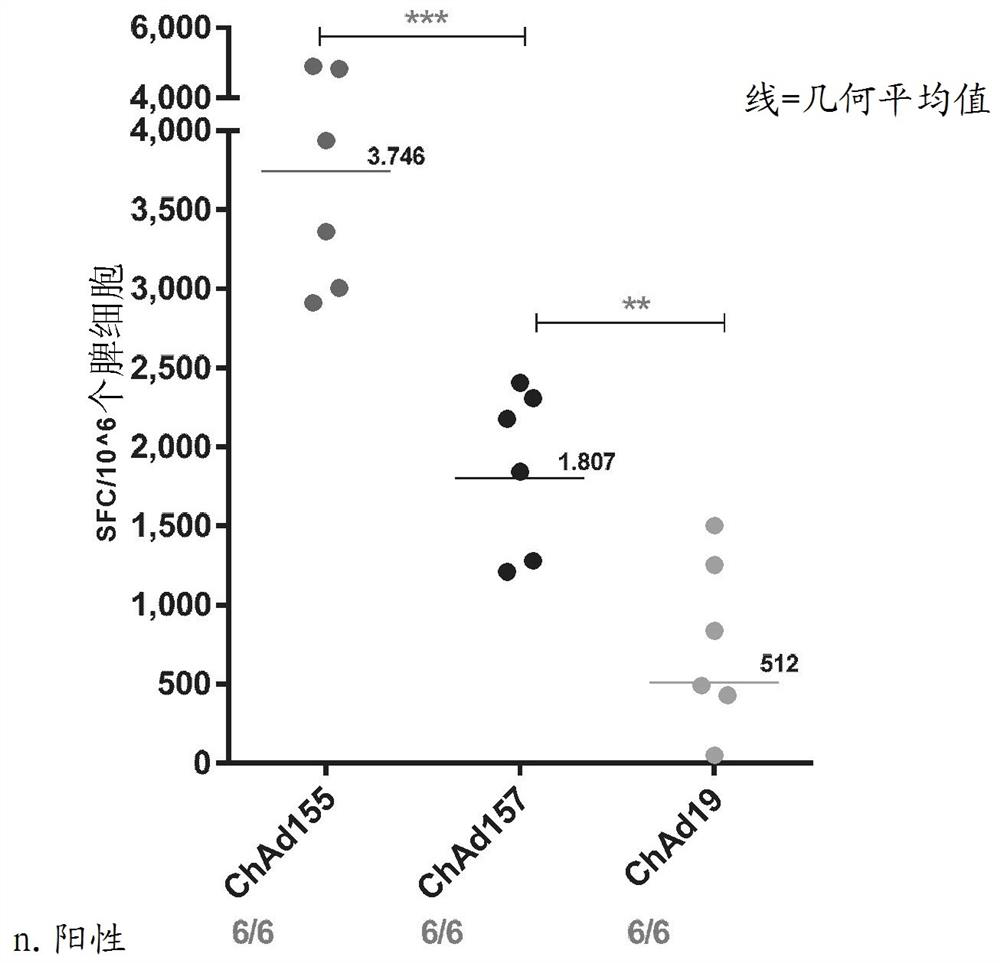
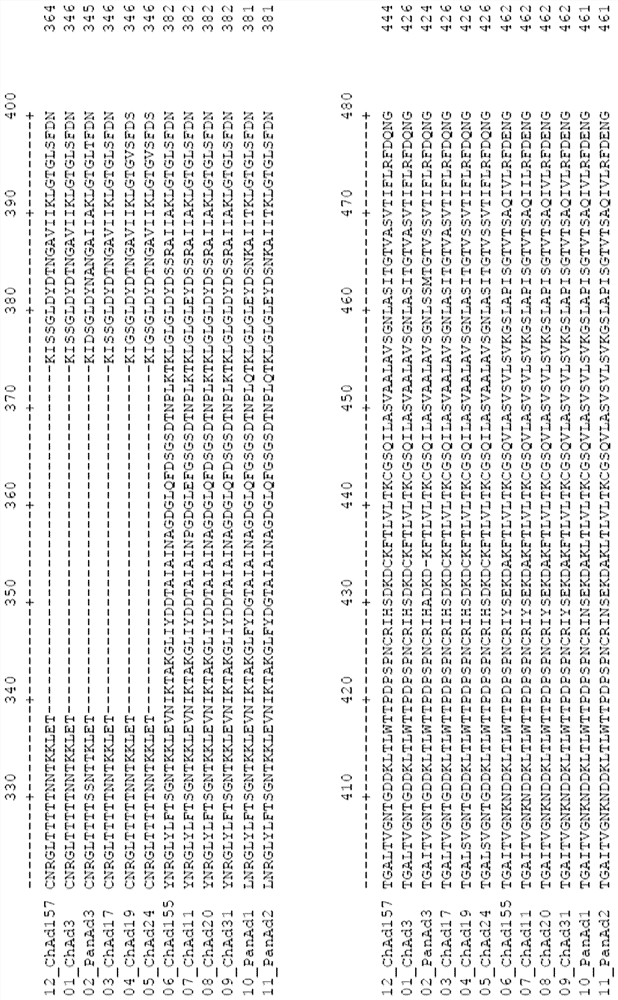
Adenovirus polynucleotides and polypeptides
A polynucleotide and adenovirus technology, applied in the field of adenovirus polynucleotides and polypeptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0360] Example 1: Isolation and vector construction of ChAd157
[0361] Using as Colloca et al. . Sci Transl Med. 2012 Jan 4;4(115):115ra2 and WO2010 / 086189 (which is incorporated herein by reference for the purpose of describing adenovirus isolation and characterization techniques) from healthy individuals in captivity in various European facilities. Isolation of 29 different wild-type chimpanzee adenoviruses from young chimpanzees.
[0362] The 29 wild-type viruses were subsequently pooled; the viral genomes of this pool were cloned by homologous recombination in E. coli BJ5183 cells using the BAC shuttle to generate a mini-library of vectors carrying deletions of the El region. A mini-library of ΔE1 vectors was transfected into the Procell 92 cell line; the rescued vectors were serially infected for 16 passages. At passage 16, viral DNA was prepared from the amplified vector and cloned by homologous recombination in E. coli BJ5183 cells using a plasmid shuttle. A popular...
Embodiment 2
[0392] Example 2: Vector Production
[0393] The productivity of ChAd157 was assessed compared to ChAd19 and ChAd155 in the Procell 92 cell line.
[0394] 2.1: Production of vectors containing the HIV Gag transgene
[0395] ChAd157 / GAG, ChAd19 / GAG, ChAd155 / GAG (ChAd157, ChAd19 and ChAd155 vectors expressing the HIV Gag transgene) were rescued and amplified in Procell 92; lysates were used to infect 1 T25 cultured in monolayer with each vector Flask of Procell 92. A multiplicity of infection (MOI) of 300 vp / cell was used and infection was performed in the presence of tetracycline because ChAd19 / GAG lacks transcriptional control mediated by insertion of the TetO operator in the hCMV promoter. Infected cells were harvested when the complete cytopathic effect was evident (48 hours after ChAd157 / GAG and ChAd155 / GAG infection and 5 days after ChAd19 / GAG infection); Infected cells release virus, and the lysate is clarified by centrifugation. Clarified lysates were quantified by...
Embodiment 3
[0404] Example 3: Transgene Expression Levels
[0405] 3.1: Expression level of HIV Gag transgene
[0406] Expression levels were compared in parallel experiments by infecting HeLa cells with ChAd19, ChAd155 and ChAd157 vectors containing the HIV Gag transgene.
[0407] HeLa cells were seeded in 35 mm dishes and infected with ChAd19 / GAG, ChAd157 / GAG and ChAd155 / GAG purified viruses using MOI = 250 vp / cell. Supernatants of infected HeLa cells were harvested 48 hours after infection and the production of secreted HIV GAG protein was quantified by using a commercial ELISA kit (HIV-1 p24 ELISA kit, PerkinElmer Life Science). Quantification was performed by using the HIV-1 p24 antigen standard curve according to the manufacturer's instructions.
[0408] Results expressed in pg / ml GAG protein are shown in Figure 7 middle.
[0409] 3.2: Expression level of RG transgene
[0410] Western blot analysis was also performed to assess the expression of rabies glycoprotein provided...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com