Method for preparing 2, 6-naphthalene dicarboxylic acid by oxidizing 2, 6-diisopropylnaphthalene
A technology of diisopropylnaphthalene and naphthalene dicarboxylic acid, applied in chemical instruments and methods, preparation of organic compounds, catalytic reactions, etc., can solve the problem of low purity of 2,6-NDA and low yield of 2,6-NDA problem, to achieve the effect of reducing difficulty and good technical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
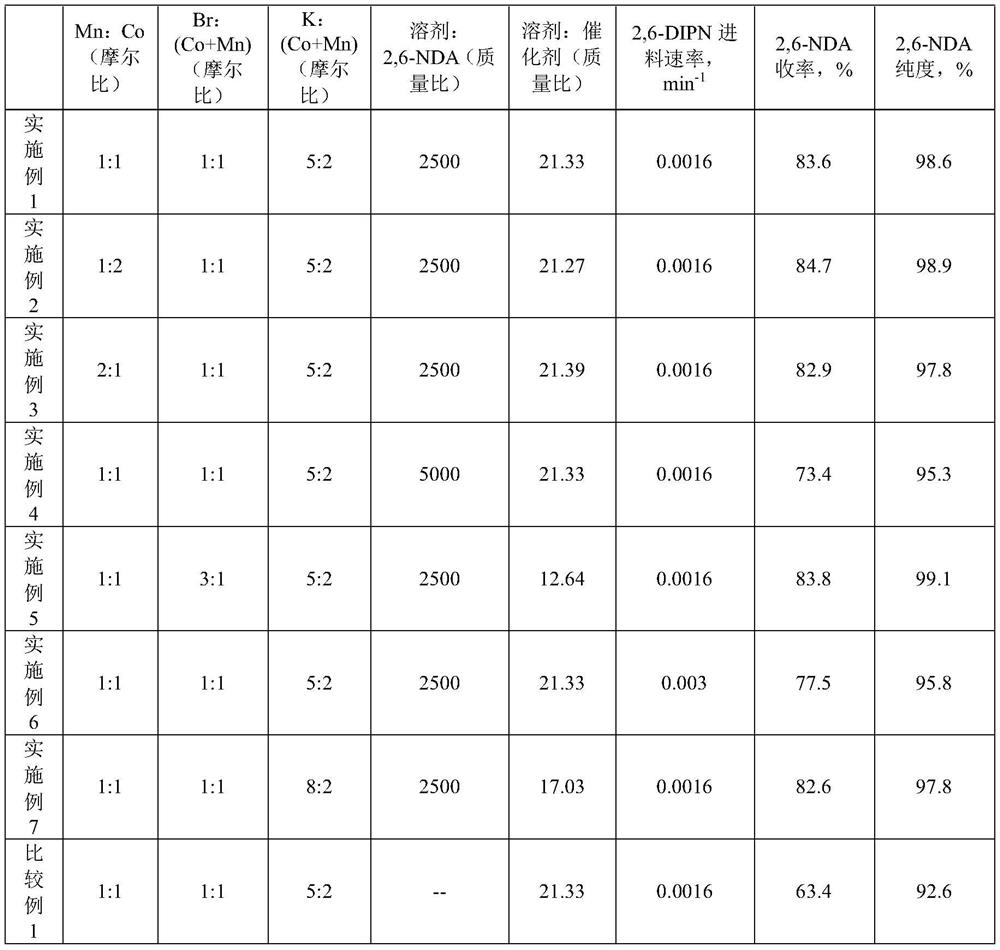
Examples
Embodiment 1
[0028] 124.5gCo(OAc) 2 4H 2 O, 122.5gMn(OAc) 2 4H 2 O, 119gKBr, 147.2gCH 3 COOK, 2g of 2,6-naphthalene dicarboxylic acid and 5000g of acetic acid were mixed and added into the reactor, then the stirring was started and the temperature was raised to 200°C, the pressure of the reactor was controlled at 2.75MPa, and 1000g of 2,6-diisopropylnaphthalene was heated to Molten state, enters in the reaction kettle with the speed of 8g / min then, feeds the air of 80L / min simultaneously to react, after feeding finishes, feeds air under the maintenance reaction temperature and pressure to continue reaction 1h, after reaction finishes, will contain The reaction product mixture of crude 2,6-naphthalene dicarboxylic acid was vacuum filtered, and washed with acetic acid at 60°C and water at 80°C respectively. The amount of acetic acid for washing was 1000g, and the amount of washing water was 1000g. After sampling and drying, analyze the obtained 2, The purity and yield of 6-naphthalene di...
Embodiment 2
[0031] 166.05gCo(OAc) 2 4H 2 O, 81.7gMn(OAc) 2 4H 2 O, 119gKBr, 147.2gCH 3 COOK, 2g of 2,6-naphthalene dicarboxylic acid and 5000g of acetic acid were mixed and added into the reactor, then the stirring was started and the temperature was raised to 200°C, the pressure of the reactor was controlled at 2.75MPa, and 1000g of 2,6-diisopropylnaphthalene was heated to Molten state, enters in the reactor with the rate of 8g / min then, feeds the air of 80L / min simultaneously and reacts, and after feeding finishes, maintains reaction temperature and pressure and continues reaction 1h, and after reaction finishes, will contain crude 2,6 -The reaction product mixture of naphthalene dicarboxylic acid was vacuum filtered, and washed with acetic acid at 60°C and water at 80°C respectively. The amount of acetic acid for washing was 1000g, and the amount of washing water was 1000g. The obtained 2,6-naphthalene dicarboxylic acid was analyzed after sampling and drying. The purity of formic a...
Embodiment 3
[0034] 83.03gCo(OAc) 2 4H 2 O, 163.4gMn(OAc) 2 4H 2 O, 119gKBr, 147.2gCH 3 COOK, 2g of 2,6-naphthalene dicarboxylic acid and 5000g of acetic acid were mixed and added into the reactor, then the stirring was started and the temperature was raised to 200°C, the pressure of the reactor was controlled at 2.75MPa, and 1000g of 2,6-diisopropylnaphthalene was heated to Molten state, enters in the reactor with the rate of 8g / min then, feeds the air of 80L / min simultaneously and reacts, and after feeding finishes, maintains reaction temperature and pressure and continues reaction 1h, and after reaction finishes, will contain crude 2,6 -The reaction product mixture of naphthalene dicarboxylic acid was vacuum filtered, and washed with acetic acid at 60°C and water at 80°C respectively. The amount of acetic acid for washing was 1000g, and the amount of washing water was 1000g. The obtained 2,6-naphthalene dicarboxylic acid was analyzed after sampling and drying. The purity of formic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com