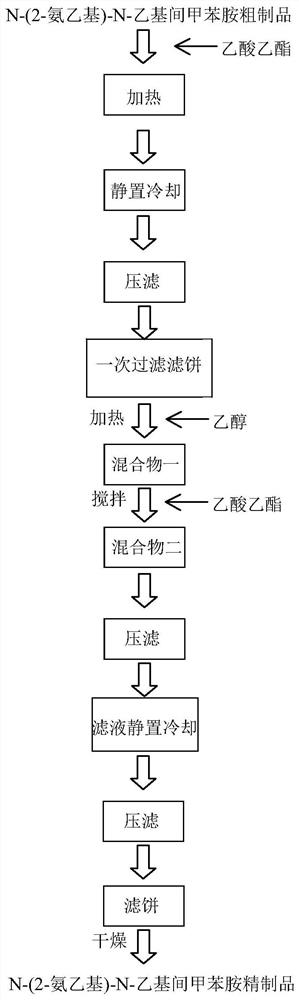
Purification method of N-(2-aminoethyl)-N-ethyl m-toluidine
A purification method and technology of toluidine, applied in amino compound purification/separation, organic chemistry, etc., can solve the problems of high equipment requirements for purification, harsh conditions for purification, etc., and achieve the effect of reducing difficulty and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0042] Preparation method: Add 0.1mol (13.52g) N-ethyl-m-toluidine, 0.11mol (22.54g) 2-bromoethylamine hydrobromide to a 250mL three-necked flask equipped with a thermometer, condenser and magnetic stirrer Salt, 0.07mol (7g) calcium carbonate and 7.5ml water, then stir the material in the flask at a speed of 300r / min, and raise the temperature to 140°C, reflux for 4h, and then naturally cool to room temperature to obtain a purity of 92.70%N - 50.2 g of crude product of (2-aminoethyl)-N-ethyl-m-toluidine.
preparation example 2
[0044]Preparation method: Add 0.1mol (13.52g) N-ethyl-m-toluidine, 0.11mol (22.54g) 2-bromoethylamine hydrobromide to a 250mL three-necked flask equipped with a thermometer, condenser and magnetic stirrer Salt, 0.07mol (7g) calcium carbonate and 7.5ml water, then stir the material in the flask at a speed of 300r / min, and raise the temperature to 140°C, reflux for 4h, and then naturally cool to room temperature to obtain a purity of 90% N - 50 g of a crude product of (2-aminoethyl)-N-ethyl-m-toluidine.
preparation example 3
[0046] Preparation method: Add 0.1mol (13.52g) N-ethyl-m-toluidine, 0.11mol (22.54g) 2-bromoethylamine hydrobromide to a 250mL three-necked flask equipped with a thermometer, condenser and magnetic stirrer Salt, 0.07mol (7g) calcium carbonate and 7.5ml water, then stir the material in the flask at a speed of 300r / min, and raise the temperature to 140°C, reflux for 4h, and then naturally cool to room temperature to obtain a purity of 93% N - 49.5 g of crude product of (2-aminoethyl)-N-ethyl-m-toluidine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com