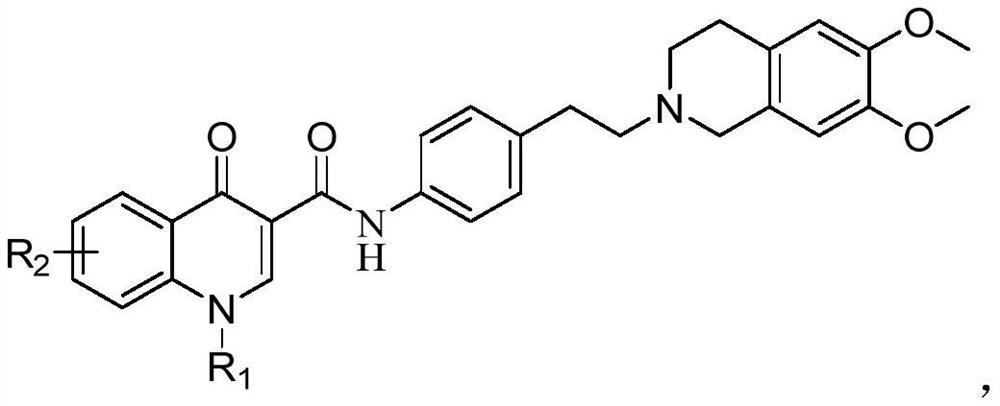
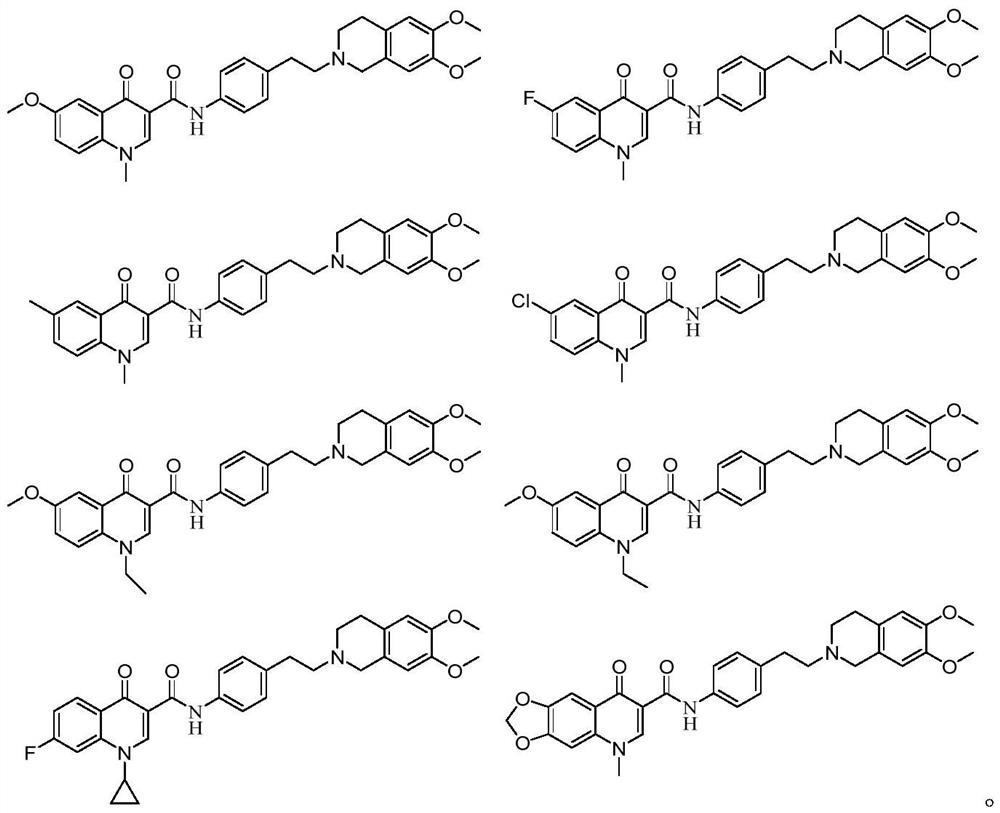
4-hydroxyquinoline derivative as well as preparation method and application thereof in antitumor drugs
A technology of hydroxyquinolines and derivatives, applied in antitumor drugs, drug combinations, organic chemistry, etc., can solve the problem of lack of P-gp specificity, low affinity of P-gp inhibitors, insufficient to produce obvious inhibitory effect, strong side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
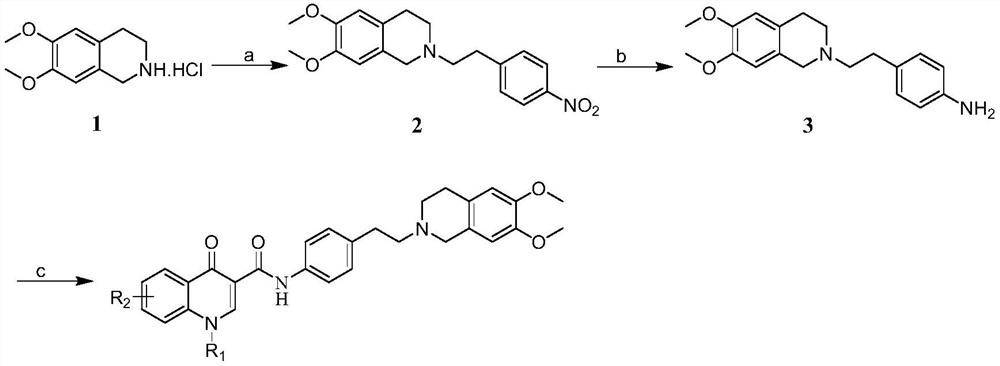
[0030] Synthesis of Step 1 Intermediate 2
[0031] 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (5.00g, 21.77mmol), 4-nitrophenethyl bromide (5.51g, 23.94mmol) and Potassium carbonate (7.52g, 54.42mmol) was dissolved in 100mL of acetonitrile, and then heated to reflux for reaction. After 18 hours, TLC detected that the reaction was complete. The heating was stopped, and the temperature was lowered to room temperature. A solid was precipitated, and 6.75 g of a yellow solid product was obtained by filtration, with a yield of 90.57%. The next reaction was carried out directly without purification.
[0032] Synthesis of Step 2 Intermediate 3
[0033] Intermediate 2 (6.0 g, 17.52 mmol) was dissolved in 80 mL of ethanol, palladium-carbon (10%, 0.6 g) was slowly added, and reacted at room temperature for 16 h under hydrogen atmosphere. The completion of the reaction was detected by TLC, the palladium-carbon was filtered off, and the filtrate was concentrat...
Embodiment 2
[0038]
[0039] 1 H-NMR (400MHz, DMSO-d 6 )δ13.30(s,1H),11.02(s,1H),8.74(s,1H),7.70(d,J=8.4Hz,2H),7.34–7.28(m,2H),7.11(d,J =8.4Hz, 2H), 7.01(d, J=8.1Hz, 1H), 6.66(s, 1H), 6.61(s, 1H), 3.69(s, 6H), 3.54(s, 2H), 2.84-2.77 (m,2H),2.66-2.52(m,6H).ESI-MS m / z:502.4[M+H] + .
Embodiment 3
[0041]
[0042] 1 H-NMR (400MHz, DMSO-d 6 )δ13.28(s,1H),11.04(s,1H),8.72(s,1H),7.71(d,J=8.4Hz,2H),7.52(d,J=1.4Hz,1H),7.30( dt,J=8.4,1.5Hz,1H),7.11(d,J=8.4Hz,2H),6.66(s,1H),6.62(s,1H),6.48(d,J=8.0Hz,1H)3.70 (s,6H),3.55(s,2H),2.84-2.76(m,2H),2.66-2.54(m,6H),2.42(s,3H).ESI-MS m / z:498.6[M+H ] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com