Tetrandrine-loaded liposome preparation as well as preparation method and application thereof
A technology of tetrandrine and liposome preparations, which is applied in the field of tetrandrine-containing liposome preparations and its preparation, and can solve the problems of large impact on intraocular pressure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
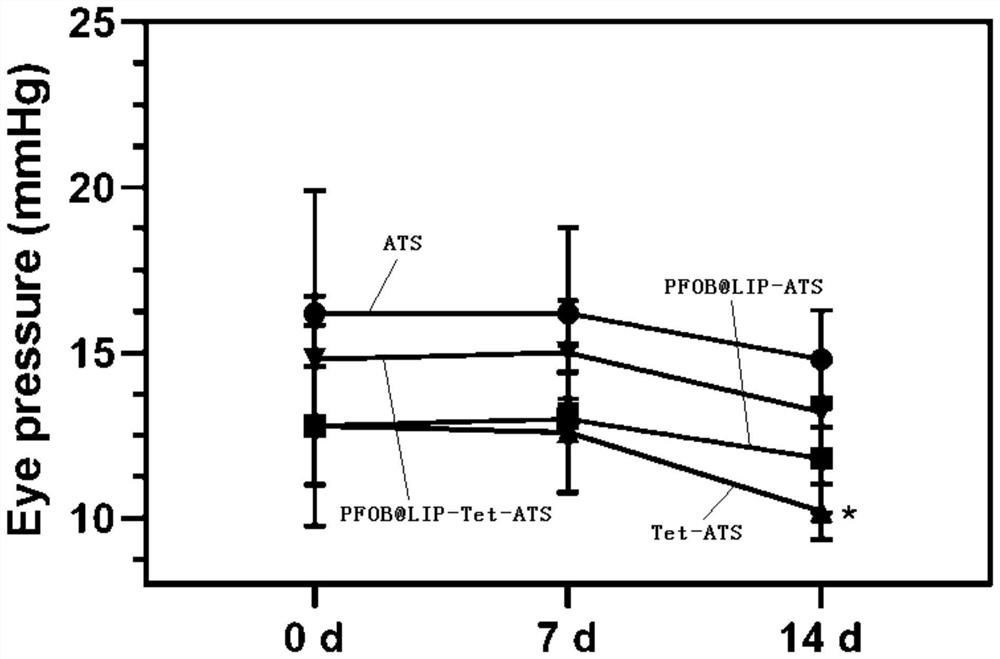
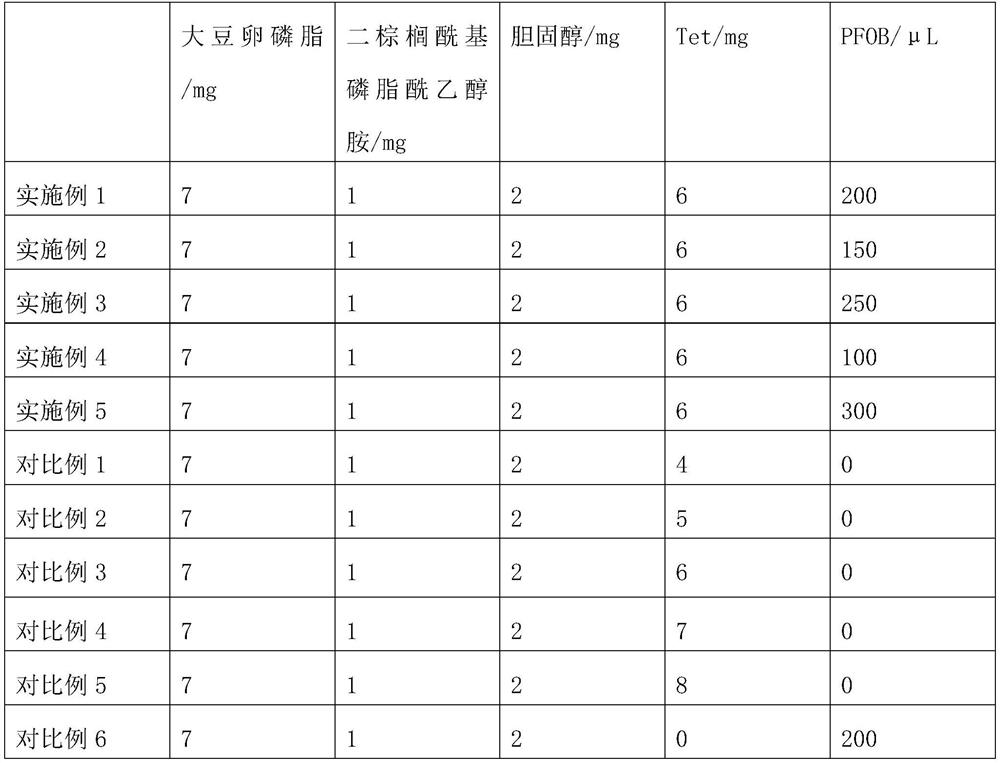
[0027] A tetrandrine-loaded liposome preparation, comprising the following raw materials: soybean lecithin, dipalmitoyl phosphatidylethanolamine, cholesterol, tetrandrine and perfluorooctyl bromide, wherein soybean lecithin and dipalmitoyl phospholipid The mass ratio of the sum of the mass of acyl ethanolamine, cholesterol, and tetrandrine is 4:1:2.5~3.5, and the ratio of the amount of perfluorooctyl bromide to the amount of tetrandrine is 150μL:6mg~250μL : 6 mg.
[0028] A preparation method for tetrandrine-loaded liposome preparation, comprising the following steps:
[0029] S1. Dissolving soybean lecithin, dipalmitoyl phosphatidylethanolamine, cholesterol and tetrandrine in a solvent, and rotary evaporation to obtain a uniform thin liquid film; in this embodiment, soybean lecithin, dipalmitoyl phosphatidylethanolamine, Cholesterol and tetrandrine were taken 7mg, 1mg, 2mg, 6mg respectively, the solvent was chloroform, and the amount of chloroform was 10mL; the rotation spee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com