Brefeldin A derivative as well as preparation method and application thereof
A technology of feldspar and its derivatives, which is applied in the field of medicine to achieve broad research value and the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
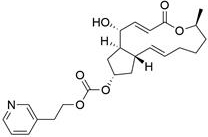
[0223] Example 1: BFA-7-(pyridin-3-ylmethyl)carbonate (Compound 1)
[0224]
[0225] Under nitrogen protection, BFA (50 mg, 1 eq) and triphosgene (50 mg, 1 eq) were dissolved in 3 ml of dry acetone, stirred at room temperature for 10 min, and then slowly added dropwise 2 ml of DMAP (100 mg, 5 eq) in acetone After stirring for 5 min, the system was transferred to an oil bath at 65 °C, continued to stir for 15 min, and slowly added dropwise 1 ml of nicotinyl alcohol (0.15 ml, 8 eq) in acetone solution, and continued stirring for 2 h after the dropwise addition. The reaction solution was concentrated under reduced pressure, and the crude extract was purified by reverse-phase silica gel column chromatography and HPLC preparation twice to obtain a colorless oily liquid. Yield 30%. 1 H NMR (600 MHz, CDC1 3 ) δ = 8.61 – 8.49 (m, 2H), 7.69 (dt, J=7.8, 2.0, 1H), 7.34 – 7.26 (m, 2H), 5.89 (dd, J=15.6, 1.9, 1H), 5.67 (ddd ,J=15.0, 10.2, 4.6, 1H), 5.18 (dd, J=15.2, 9.4, 1H), 5.10 (s...
Embodiment 2
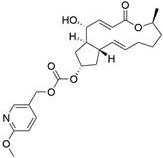
[0226] Example 2: BFA-7-(pyridin-2-ylmethyl)carbonate (Compound 2)
[0227]
[0228] Referring to the preparation method of Example 1, a colorless oily liquid was obtained. The yield was 41%. ESIMS m / z 416.21 [M + H] + .
Embodiment 3
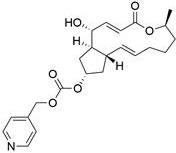
[0229] Example 3: BFA-7-(pyridin-4-ylmethyl)carbonate (Compound 3)
[0230]
[0231] Referring to the preparation method of Example 1, a colorless oily liquid was obtained. The yield was 38%. ESIMS m / z 416.21 [M + H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com