Application of levistilide A in preparation of medicine for in-vitro amplification of human hematopoietic stem cells
A technology of angelica lactone and in vitro amplification, applied in the field of application of angelica lactone A in the preparation of drugs for in vitro expansion of human hematopoietic stem cells, can solve problems such as unreported, and achieve good self-renewal and self-renewal Promoted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of small molecule compound Levistilide A (Levistilide A), its structure is:
[0034]
[0035] The physical and chemical identification of the small molecule compound angelicalide A, including:
[0036] Purity determined by high performance liquid chromatography (HPLC): 98.8%.
[0037] H NMR ( 1 H NMR) (400MHz, CDCl3) δ7.34(d, J=6.6Hz, 1H), 5.06(t, J=7.9Hz, 1H), 4.99(t, J=7.5Hz, 1H), 3.24(d, J=8.9Hz, 1H), 2.98(dd, J=6.5, 2.1Hz, 1H), 2.54(t, J=7.7Hz, 1H), 2.28(q, J=7.6Hz, 2H), 2.24–2.14( m,3H),2.13–1.98(m,2H),1.97–1.82(m,2H),1.63–1.40(m,6H),1.30(ddd,J=12.2,4.7,2.6Hz,1H),0.98– 0.87(m,6H).
[0038] Carbon NMR ( 13 C NMR) (100MHz, CDCl3) δ168.6, 165.0, 155.1, 150.6, 148.1, 142.2, 134.3, 126.7, 112.3, 108.7, 47.7, 41.7, 41.6, 38.4, 31.2, 29.1, 28.1, 27.6, 25.9, 22.4, 2 19.9, 14.1, 13.9.
[0039] High resolution mass spectrometry HRMS (ESI) determination of C 24 h 29 o 4 + [M+H] + :381.2060, found 381.2062.
[0040] A kind of small molecular compound neo...
Embodiment 2
[0047] Compound effect of embodiment 2 different concentrations
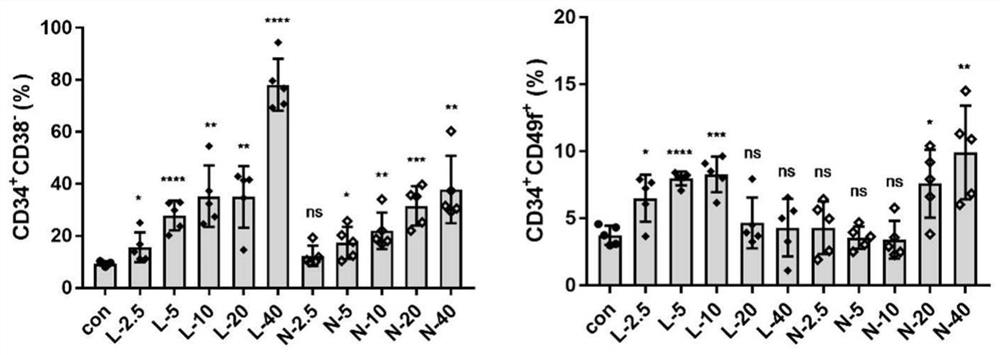
[0048] Freshly isolated human cord blood CD34 + Cells were resuspended in culture medium (Gibco Iscove’s Modified Dulbecco Medium+10% FBS+100ng / mL SCF+100ng / mL TPO+100ng / mL Flt3L+1%P / S) to a cell concentration of 5×10 4 Cells / mL, then spread in 96-well plate, each hole was 190 μL cell suspension, and then added 10 μL concentration gradient of 2.5, 5, 10, 20 and 40 μM Euangicalide A (L) and new cnidium Ester (N) was cultured, and 0.05% DMSO with the same content was used as a solvent control. After 7 days of culture, flow cytometric analysis was performed to detect the ratio of CD34+CD38- hematopoietic stem progenitor cells and relatively more primitive CD34+CD49f+ cells in CD34+ cells.
[0049] Result analysis: if figure 1 As shown, for the CD34+CD38- cell population, both compounds exhibited an approximate dose-dependent expansion effect, and for the CD34+CD49f+ cell population, Euangicalide A significantly e...
Embodiment 3
[0051] A method for in vitro expansion of human hematopoietic stem cells, comprising the steps of:
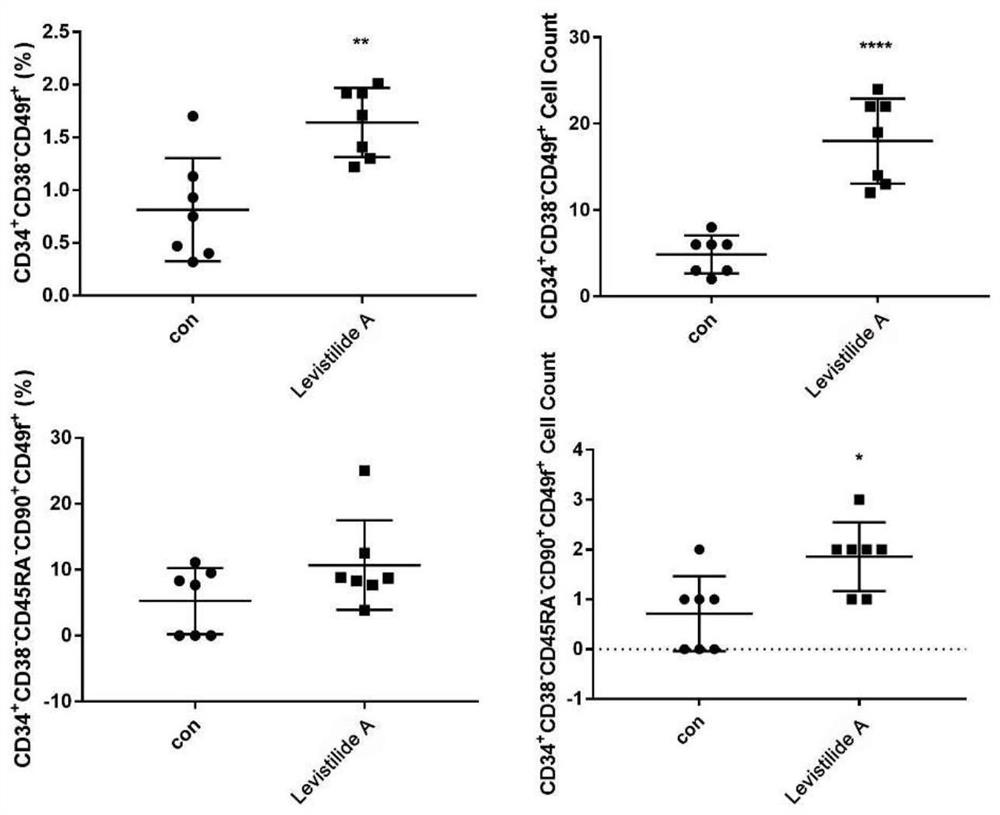
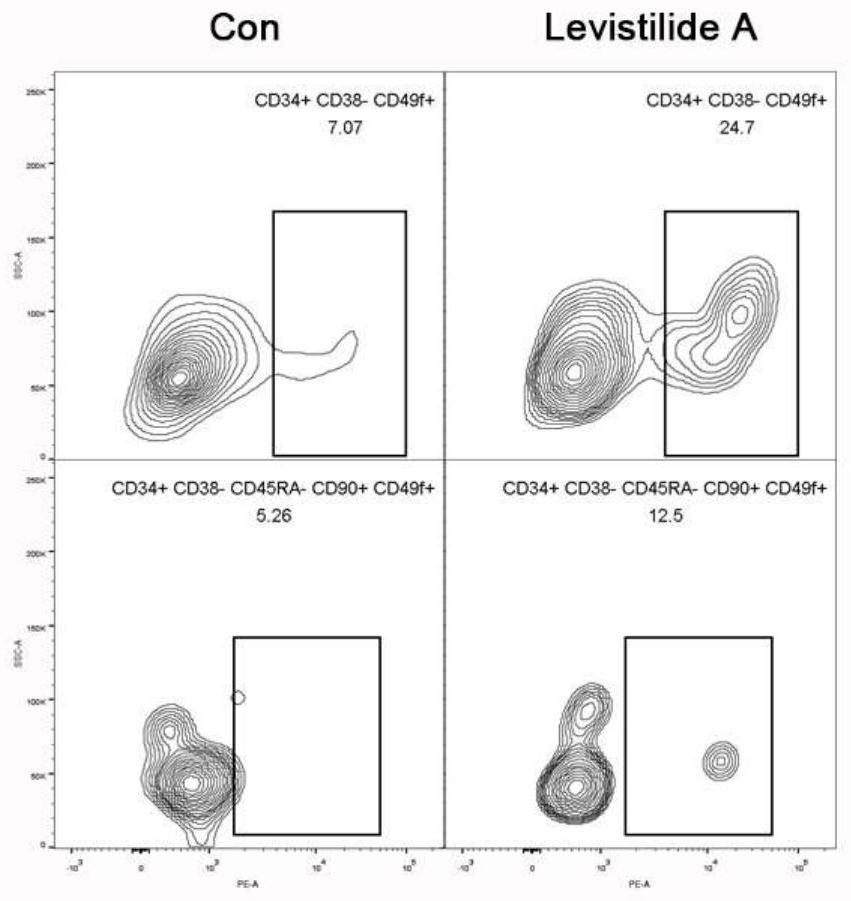
[0052] Freshly isolated human cord blood CD34 + Cells were resuspended in culture medium (Gibco Iscove’s Modified Dulbecco Medium+10% FBS+100ng / mL SCF+100ng / mL TPO+100ng / mL Flt3L+1%P / S) to a cell concentration of 5×10 4 Cells / mL, then spread in a 96-well plate, each well is 190 μL of cell suspension, and then add 10 μL of the small molecule compound of Euangicalide A diluted with the same culture medium, the final concentration of Euangicalide A 10 μM. at 37°C, 5% CO 2 Cultured in a constant temperature incubator for 7 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com