Method for detecting fudosteine and impurities and enantiomers in fudosteine tablet
A technology for enantiomers and fudosteine tablets, which is applied in the field of detection of impurity A, impurity B and dextro-isomer in fudosteine and fudosteine tablets, and can solve the problem of easily interfering with other impurities, Problems such as different retention times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
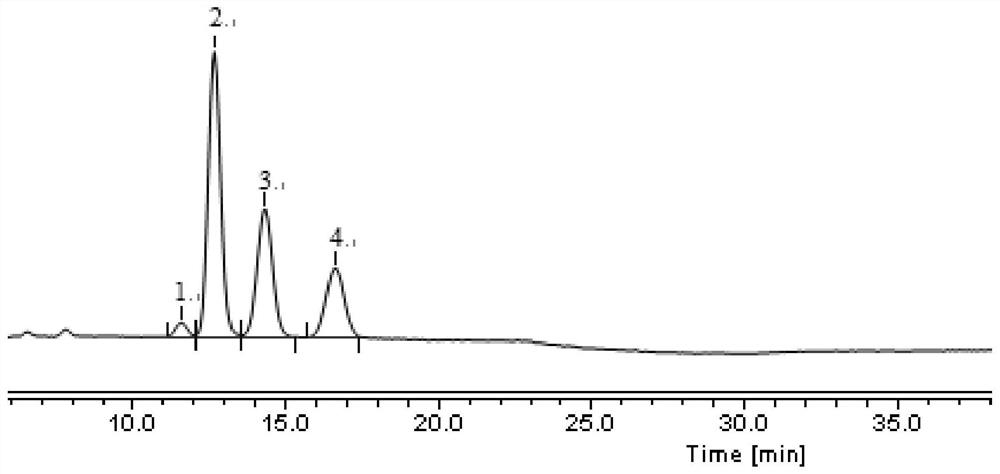
[0099] Example 1 Detection of Impurity A, Impurity B and Enantiomers in Fudosteine Raw Materials
[0100] Instrument and equipment samples:
[0101] High performance liquid chromatography: Ultimate3000
[0102] Analytical balance: Mettler Toledo XP205
[0103] pH meter: Mettler Toledo S20
[0104] Fudosteine samples: 1901001, 1901002, 1901003, produced by Jiangsu Zhengda Fenghai Pharmaceutical Co., Ltd.
[0105] Chromatographic conditions:
[0106] Chromatographic column: Silica gel coated with chiral crown ether is used as filler;
[0107] Mobile phase: use pH1.5 perchloric acid solution as the mobile phase;
[0108] Flow rate: 0.25ml / min;
[0109] Column temperature: 12°C;
[0110] Detector: UV detector, the detection wavelength is 210nm.
[0111] Detection steps:
[0112] (1) Take about 300mg of fudosteine raw material, accurately weigh it, put it in a 10ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, centrifuge, and take...
Embodiment 2
[0127] The detection of impurity A, impurity B and enantiomer in the fodosteine raw material of embodiment 2
[0128] With reference to the equipment, chromatographic conditions and detection steps in Example 1, only the sample batch number is changed to 1906001, 1906002, 1906003, the mobile phase is changed to pH1.2 perchloric acid solution in the chromatographic conditions, the flow rate is changed to 0.20ml / min, and the column Change the temperature to 15°C.
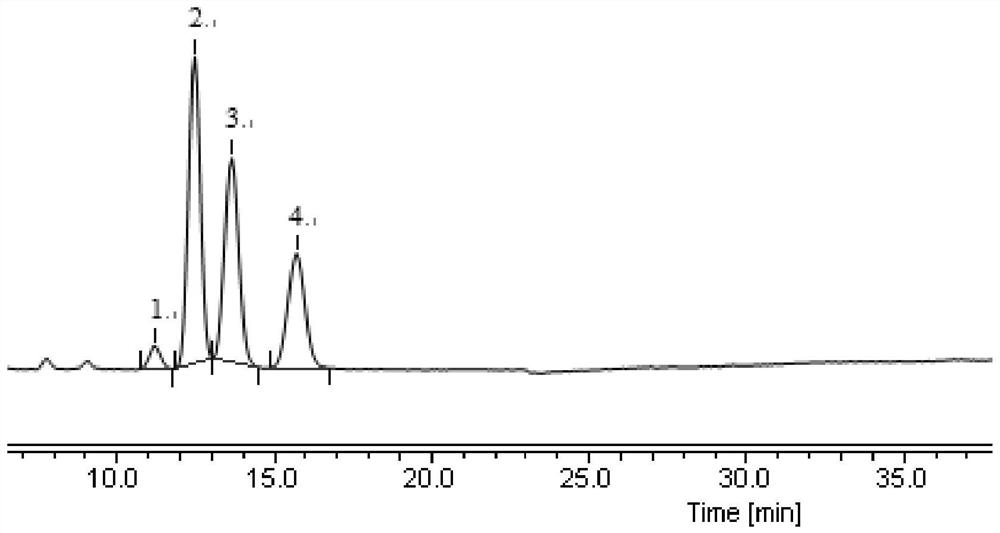
[0129] Test results: Calculated by the peak area according to the external standard method, the contents of impurity A, impurity B and dextro isomer in the three batches of samples are all no more than 0.1%, all complying with the regulations ( image 3 , Figure 4 , Table 13).
[0130] Table 13 Example 2 detection results
[0131]
[0132] Note: the calculation formula is the same as in Example 1.
Embodiment 3
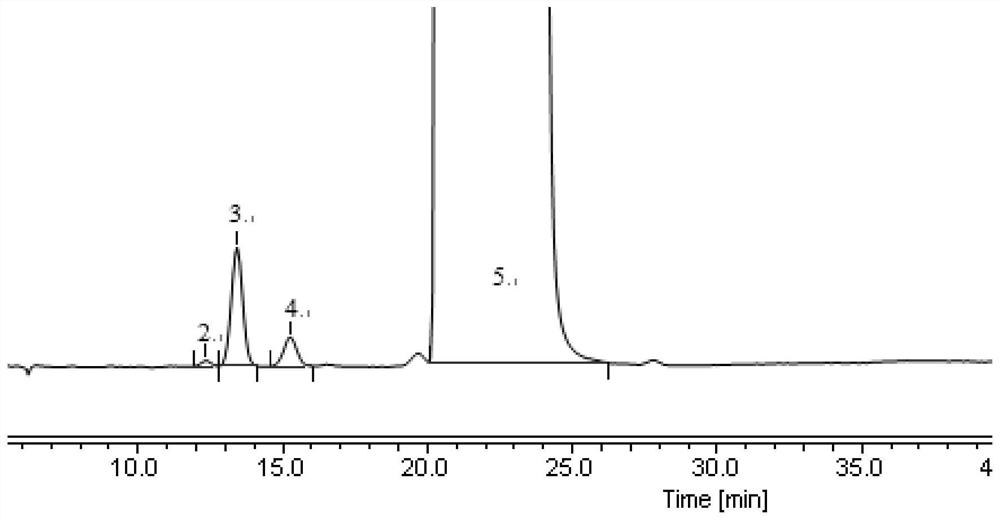
[0133] Example 3 Detection of Impurity A, Impurity B and Enantiomers in Fudosteine Tablets
[0134] Instrument and equipment samples:
[0135] High performance liquid chromatography: Ultimate3000
[0136] Analytical balance: Mettler Toledo XP205
[0137] pH meter: Mettler Toledo S20
[0138] Samples of fudosteine tablets: 1704151, 1710251, 1710261, produced by Jiangsu Zhengda Fenghai Pharmaceutical Co., Ltd.
[0139] Chromatographic conditions:
[0140] Chromatographic column: silica gel coated with chiral crown ether is used as filler;
[0141] Mobile phase: use pH1.5 perchloric acid solution as the mobile phase;
[0142] Flow rate: 0.25ml / min;
[0143] Column temperature: 10°C;
[0144] Detector: UV detector, the detection wavelength is 210nm.
[0145] Detection steps:
[0146] (1) Take fudosteine tablet and grind it into fine powder, take an appropriate amount (approximately equivalent to fudosteine 200mg), weigh it accurately, put it in a 10ml measuring bott...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com