Bumetanide injection and preparation method thereof
A technology of bumetanide and bumetanide ≤ is applied in the field of medicine, which can solve problems such as uncontrollable quality defects and cumbersome operations, and achieve the effects of high stability, simplified process and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
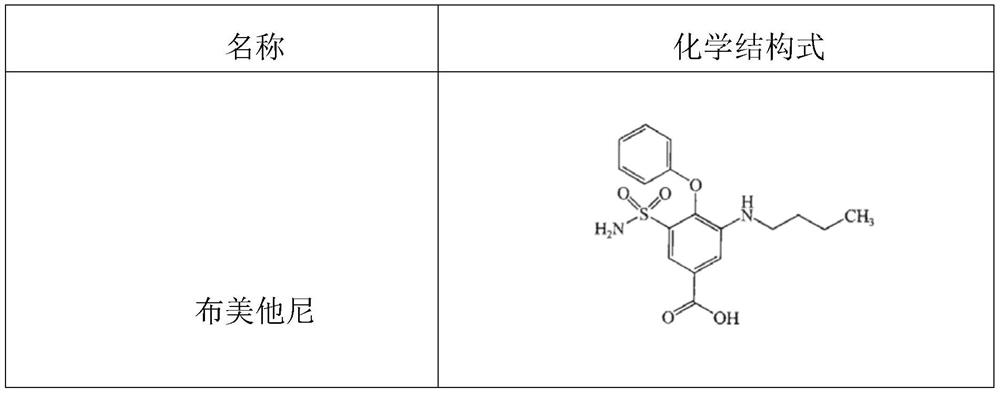
[0031] (1) Accurately weigh 0.25g of bumetanide;
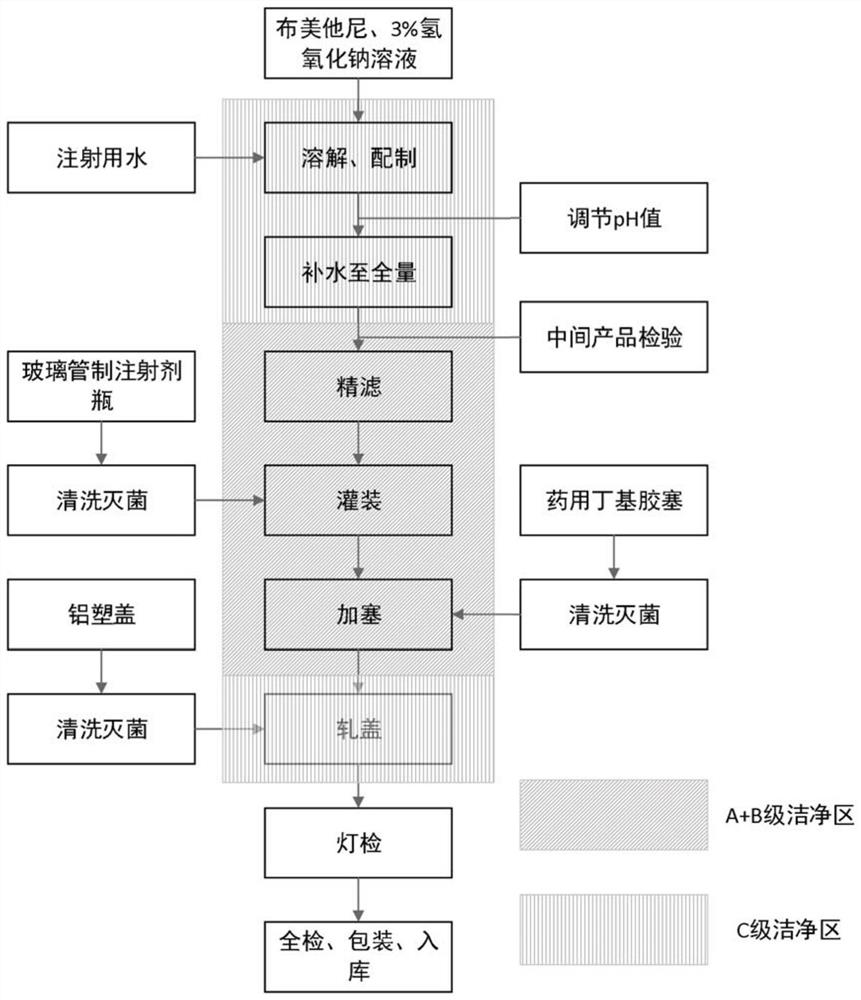
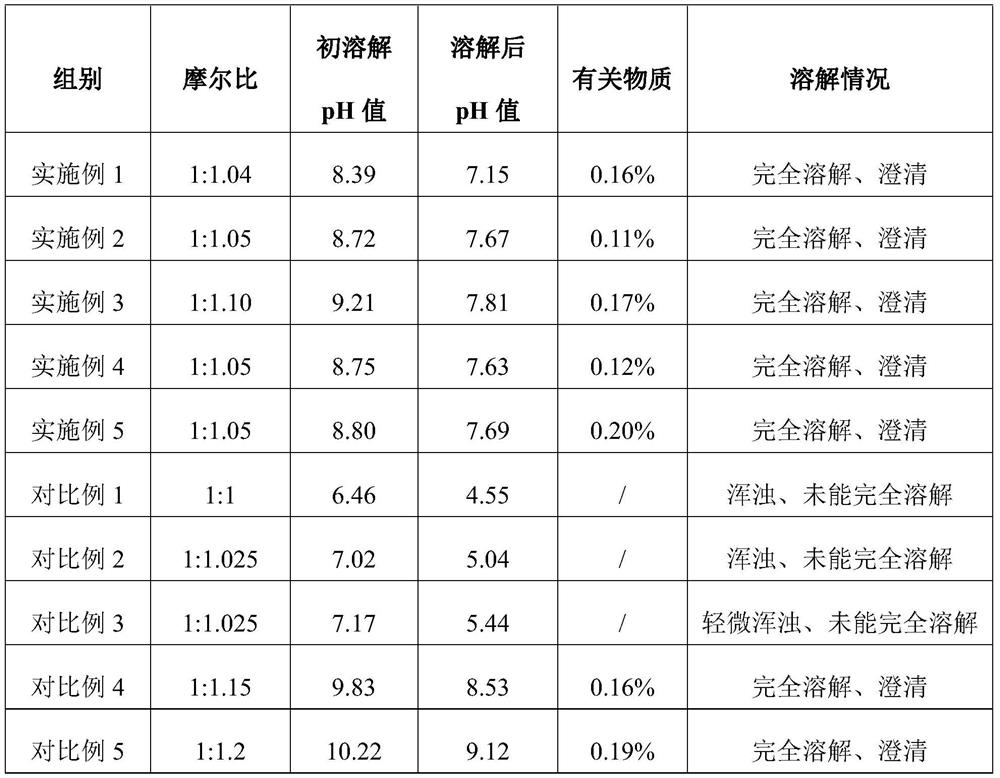
[0032] (2) Add water for injection at 8°C to bumetanide, the amount of water for injection is 90% of the total amount prepared; under stirring state, according to the molar ratio of bumetanide to sodium hydroxide 1:1.04, accurately add 0.95ml of 3% sodium hydroxide solution, and ultrasonically treat the mixed drug solution for 15 minutes. After the treatment is completed, continue to stir the mixed drug solution for 20 minutes; observe the dissolution state of bumetanide, and monitor the solution just after adding 3% sodium hydroxide solution and stirring The pH value after completion is recorded in Table 1;
[0033] (3) Add the remaining water for injection to make up to 1000ml;
[0034] (4) Use two 0.22 μm, PES material sterilization filters to carry out redundant filtration on the above solution, and the filtrate is used for standby;
[0035] (5) Divide the above-mentioned filtered solution into 2ml: 0.5mg and store it fo...
Embodiment 2
[0037] (1) Accurately weigh 0.25g of bumetanide;
[0038] (2) Add water for injection at 6°C to bumetanide, the amount of water for injection is 90% of the total amount prepared; under stirring state, according to the molar ratio of bumetanide to sodium hydroxide 1:1.05, accurately add 0.96ml of 3% sodium hydroxide solution, and sonicate the mixed drug solution for 13 minutes. After the treatment is completed, continue to stir the mixed drug solution for 20 minutes; observe the dissolution state of bumetanide, and monitor the drug solution just after adding 3% sodium hydroxide solution and stirring The pH value after completion is recorded in Table 1;
[0039] (3) Add the remaining water for injection to make up to 1000ml;
[0040] (4) Use two 0.22 μm, PES material sterilization filters to carry out redundant filtration on the above solution, and the filtrate is used for standby;
[0041] (5) Divide the above-mentioned filtered solution into 2ml: 0.5mg and store it for later...
Embodiment 3
[0043] (1) Accurately weigh 0.25g of bumetanide;
[0044] (2) Add water for injection at 2°C to bumetanide, the amount of water for injection is 90% of the total amount prepared; under stirring state, according to the molar ratio of bumetanide to sodium hydroxide 1:1.10, accurately add 1.005ml of 3% sodium hydroxide solution, and ultrasonically treat the mixed drug solution for 10 minutes. After the treatment is completed, continue to stir the mixed drug solution for 20 minutes; observe the dissolution state of bumetanide, and monitor the solution just after adding 3% sodium hydroxide solution and stirring The pH value after completion is recorded in Table 1;
[0045] (3) Add the remaining water for injection to make up to 1000ml;
[0046] (4) Use two 0.22 μm, PES material sterilization filters to carry out redundant filtration on the above solution, and the filtrate is used for standby;
[0047] (5) Divide the above-mentioned filtered solution into 2ml: 0.5mg and store it f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com