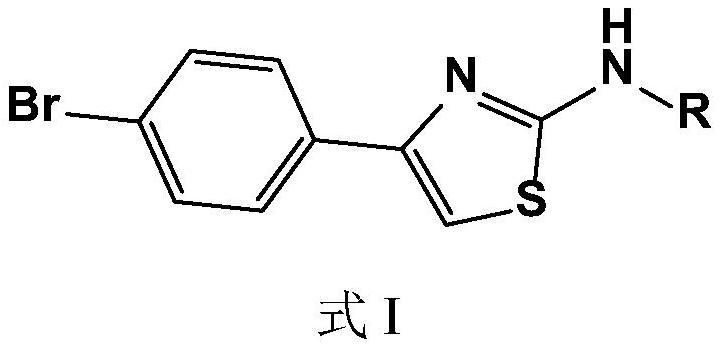
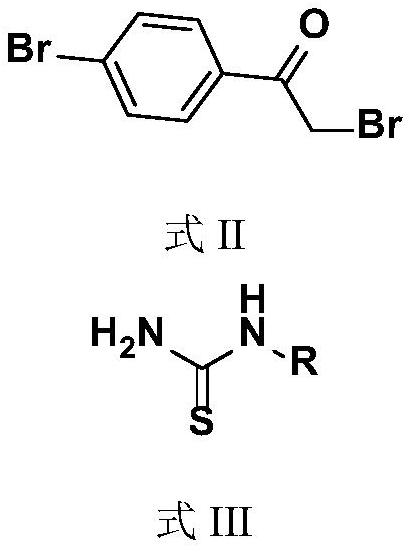
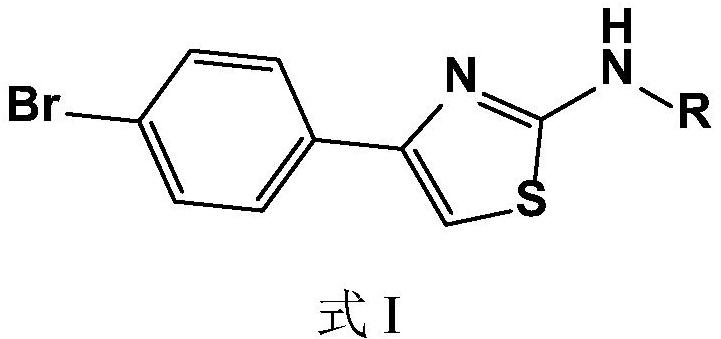
Bromine-containing compounds as antifungal agents
A technology of antibacterial drugs and compounds, applied in the field of bromine-containing compounds, can solve the problems of large dosage and frequent use, and achieve the effect of small MIC value and excellent antifungal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the synthesis of compound I1N-(4-bromophenylthiazol-2-yl)acetone hydrazone (N-(4-bromophenyl-2-thiazolyl)acetone hydrazone)
[0027] The synthetic route is as follows:
[0028]
Embodiment 2
[0031] Separation and purification of N-(4-bromophenylthiazol-2-yl)acetone hydrazone
[0032] The suspension after the reaction in Example 1 was used to filter using a Buchner funnel, fully washed with petroleum ether, and dried in vacuum. The dried solid powder was separated by forward silica gel column chromatography (forward silica gel 200-300 mesh, or 100-200 mesh), and eluted with a mixed solvent of petroleum ether and ethyl acetate (volume ratio: 3:1). The eluent containing compound I was dried under reduced pressure to remove the organic solvent to obtain the target compound.
Embodiment 3
[0033] The structure identification of embodiment 3 compound I1
[0034] HPLC was used to identify the purity of the prepared compound, and the samples with a purity greater than 98% were tested for structural identification by mass spectrometry and nuclear magnetic resonance technology, and the nuclear magnetic resonance was determined by a Bruker AVANCE DRX-500 nuclear magnetic resonance instrument; the mass spectrometer was determined by an Agilent 1946D single quadrupole mass spectrometer Determination.
[0035] The H NMR spectrum data of the target compound δ H (CDCl 3 ): 8.54(s), 7.64(d, J=8.5Hz), 7.49(d, J=8.5Hz), 6.84(s), 2.05(s), 1.84(s);
[0036] The carbon NMR spectrum data of the target compound δ H (CDCl 3 ): 169.7, 149.8, 149.5, 133.7, 131.9, 127.6, 121.8, 103.8, 25.2, 16.7;
[0037] ESI mass spectrum [M+H] of the target compound + m / z 310.0
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com