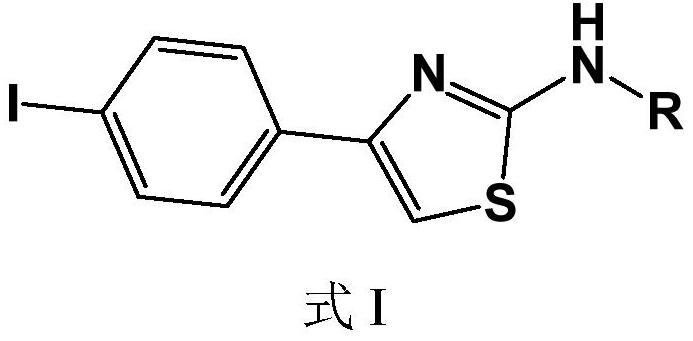
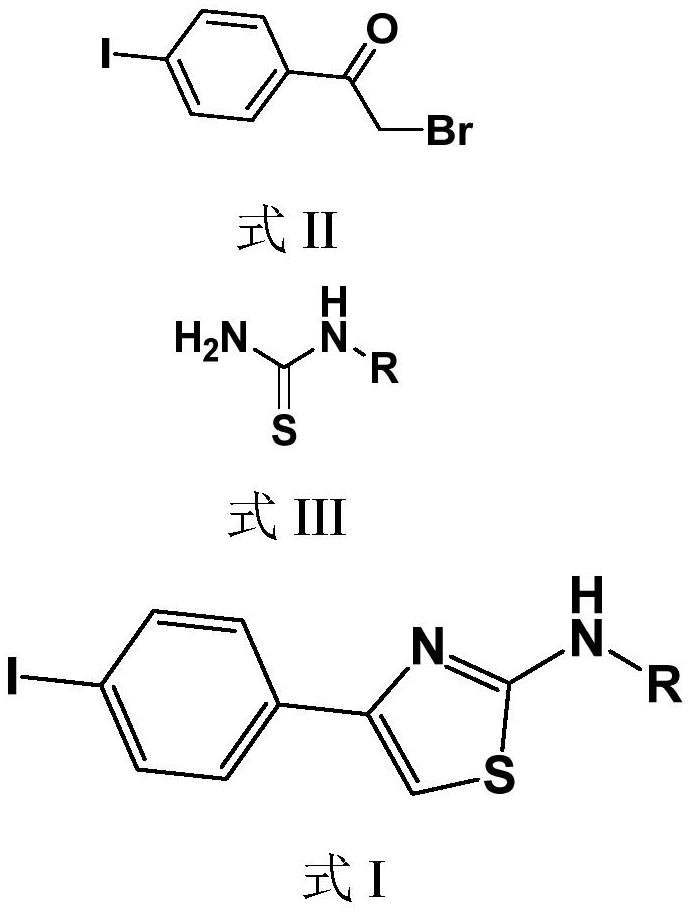
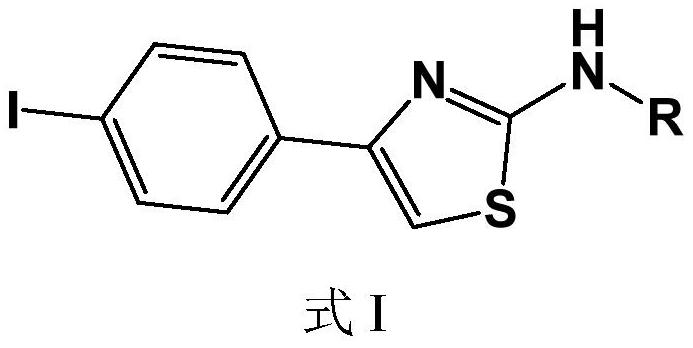
Iodine-containing compound and application thereof in fungus resistance
A compound and halide technology, applied in antifungal agents, medical preparations containing active ingredients, organic chemistry, etc., can solve the problem of increasing the dose of drugs, and achieve the effect of small MIC value and excellent antifungal effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1, N-(4-iodophenylthiazol-2-yl)acetone hydrazone (N-(4-bromophenyl-2-thiazolyl)acetone hydrazone) (also formula I1)
[0027] The synthetic route is as follows:
[0028]
Embodiment 2
[0030] Example 2, the separation and purification of N-(4-iodophenylthiazol-2-yl) acetone hydrazone obtained in Example 1
[0031] The suspension after the reaction in Example 1 was used to filter using a Buchner funnel, fully washed with petroleum ether, and dried in vacuum. The dried solid powder was separated by forward silica gel column chromatography (forward silica gel 200-300 mesh, or 100-200 mesh), and eluted with a mixed solvent of petroleum ether and ethyl acetate (volume ratio: 3:1). The eluent containing compound I was dried under reduced pressure to remove the organic solvent to obtain the target compound represented by formula I1.
Embodiment 3
[0032] Embodiment 3, the structural identification of compound I1
[0033] HPLC was used to identify the purity of the prepared compound, and the samples with a purity greater than 98% were tested for structural identification by mass spectrometry and nuclear magnetic resonance technology, and the nuclear magnetic resonance was determined by a Bruker AVANCE DRX-500 nuclear magnetic resonance instrument; the mass spectrometer was determined by an Agilent 1946D single quadrupole mass spectrometer Determination.
[0034] The H NMR spectrum data of the target compound δ H (CDCl 3 ): 7.75(d, J=8.5Hz), 7.64(d, J=8.5Hz), 7.30(s), 1.95(s), 1.93(s);
[0035] The carbon NMR spectrum data of the target compound δ C (CDCl 3 ): 170.0, 150.5, 148.9, 137.3, 134.2, 127.6, 104.2, 93.4, 24.9, 17.7;
[0036] High resolution ESI mass spectrum [M+H] of the target compound + m / z 357.9872.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com