Method for preparing sludge treatment agent and method for treating sludge
A technology for sludge treatment and sludge, which is applied in the field of preparation of sludge treatment agent and sludge treatment, can solve the problems of high cost, incomplete removal, low removal efficiency of heavy metals, etc., to achieve stability guarantee and outstanding synergistic passivation effect , Removal of heavy metals and significant effect of passivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Hereinafter, the inventive concept will be described in detail in conjunction with the accompanying drawings. However, the following specific embodiments are only used to fully convey the inventive concept to those skilled in the art, and do not limit the scope of the present invention. The scope of the invention is defined by the appended claims and their equivalents.
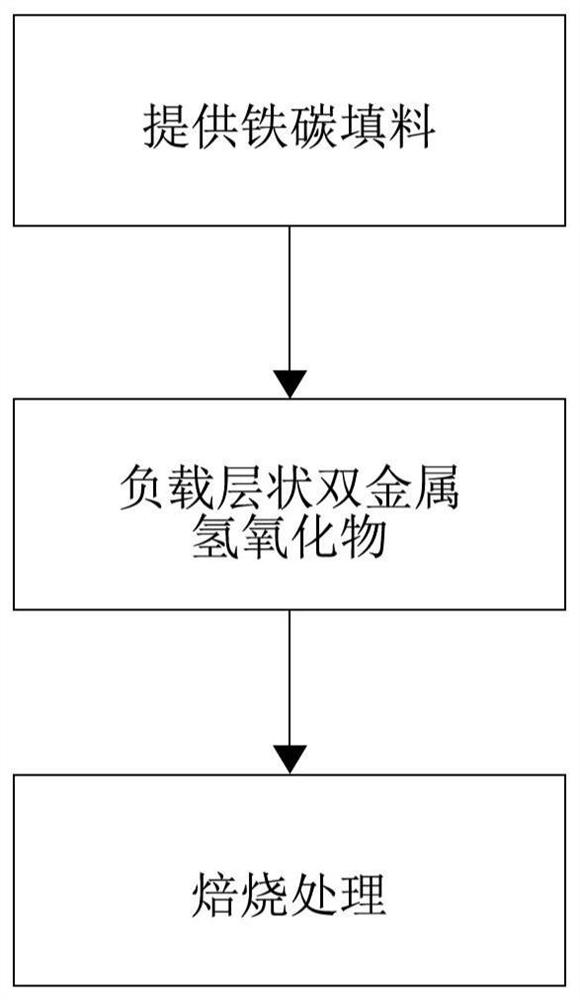
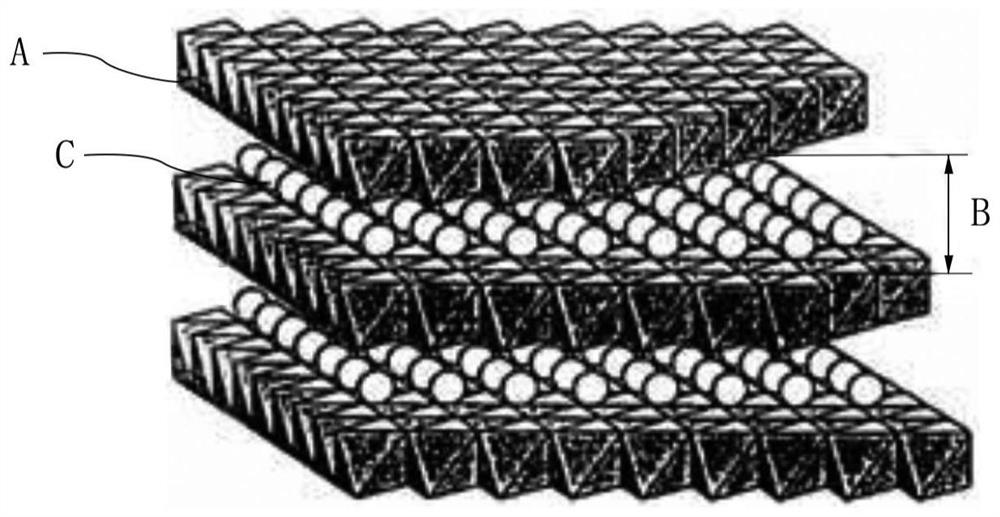
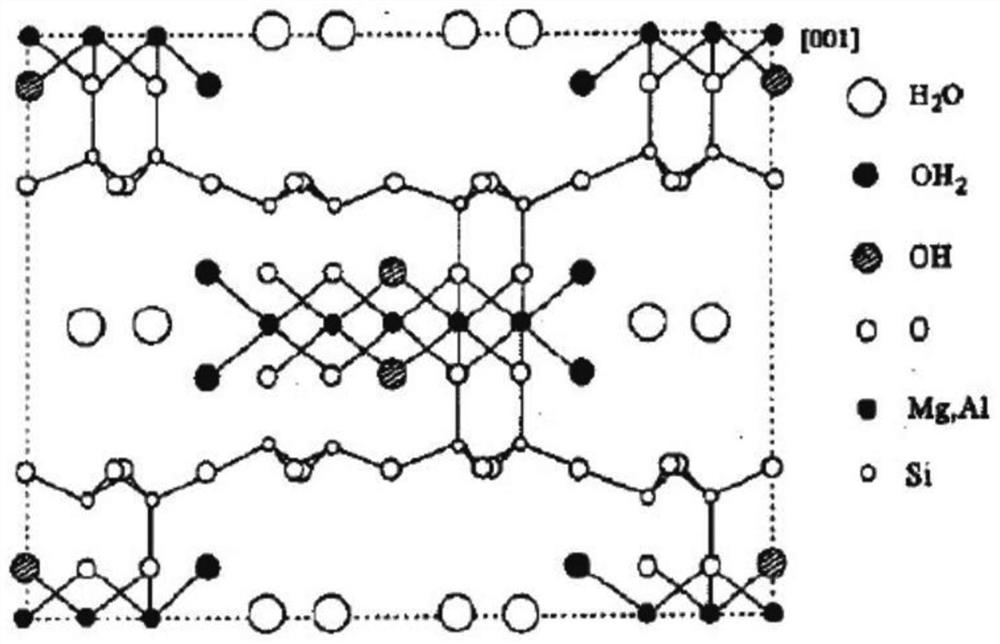
[0029] A large amount of sludge is produced during sewage treatment. Sludge is composed of bacterial micelles formed by various microorganisms, organic and inorganic substances adsorbed and water, including a large amount of heavy metals. In order to fully utilize sludge, it is necessary to remove heavy metals included in the sludge. To achieve this purpose, the present invention mainly utilizes a sludge treatment agent loaded with layered bimetallic oxides on iron-carbon fillers to achieve efficient and low-cost removal of heavy metals in sludge.
[0030] The sludge treatment agent according to the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com