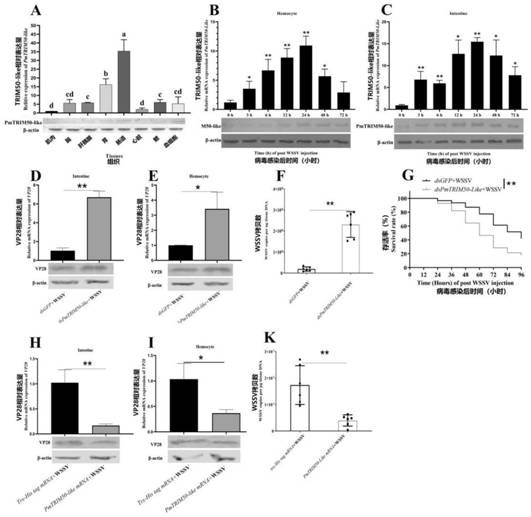
E3 ubiquitin ligase trim50-like protein of Penaeus monodon and its application
A technology of ubiquitin ligase and Penaeus monodon, which is applied in the direction of ligase, peptide/protein components, enzymes, etc., to reduce the amount of replication and improve the survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Spotstown to shrimp E3 ubiquitin ligase Trim50-Like protein
[0035] (1) Extraction of total RNA
[0036] Take the fresh healthy spots to tissue the shrimp stocks, and the total RNA was extracted according to the Qiagen Mini kit, and the total RNA was extracted and the genome was removed using DNase.
[0037] (2) Preparation of full length cDNA template
[0038] PRIMEScript TM II 1st Strand CDNA Synthesis Kit (Takara) Manual Preparation Full-length CDNA Template, 1 μL of total RNA is added 1 μL Oligo DT Primer, 1 μL DNTP MIXTURE (10mmeach), plus RNase Free DDH 2 O Suppleed to 10 μL. Mix the above solution, centrifuge, 65 ° C for 65 ° C, 5 min after 5 min, and cool the template RNA after 5 min. The following reagents were sequentially added: 4 μL5 × primescriptii, 0.5 μL inhibitor (40U / μL), 1 μL Primescript II Rtase (200U / μL) and RNASefree DDH 2 O. The following react procedures were carried out: 30 ° C 10 min, 42 ° C 15 min, 95 ° C for 5 min, and 4 ° C were co...
Embodiment 2
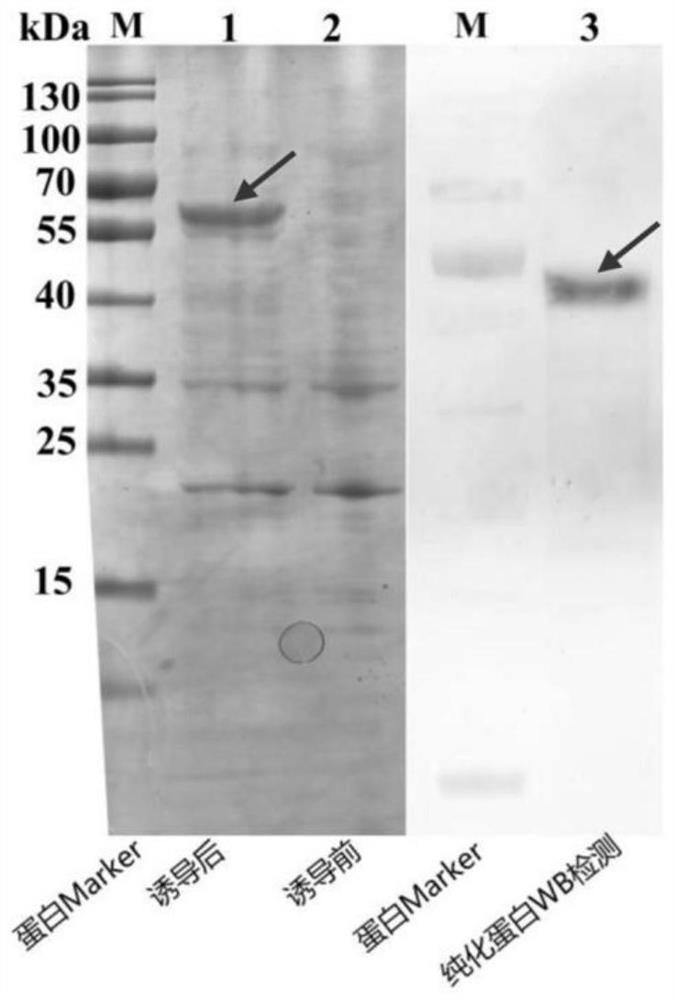
[0063] Example 2 TRIM50-LIKE protein polyclonal antibody preparation
[0064] The purified TRIM50-LIKE recombinant protein (about 100-200 μg) was fully mixed with 3 ml complete Freund adjuvant, and 8 mice were injected at a multi-point injection at a mouse skin. . Immunization 3 times after the first injection of 3 weeks. The reinforcing amount of 10-20 μg protein is used to emulsify using an incomplete Frego adjuvant. Each injection is 10 days. After the fourth injection, mice were removed from the eye, and the blood was allowed to stand for 1 h at 37 ° C for 1 h, and 4000 rpm was centrifuged for 10 min, and the upper polyhytraminated serum was absorbed, and the dispensing was stored in -80 ° C.
[0065] Such as image 3 As shown, the above-mentioned osmium shrimp Trim50-LIKE protein polyclonal antibody can specifically bind to the pestinalized Trim50-LIKE protein of the plaque, and the different tissues of three healthy shrimps utilize fluorescent quantitative PCR and protein imm...
Embodiment 3
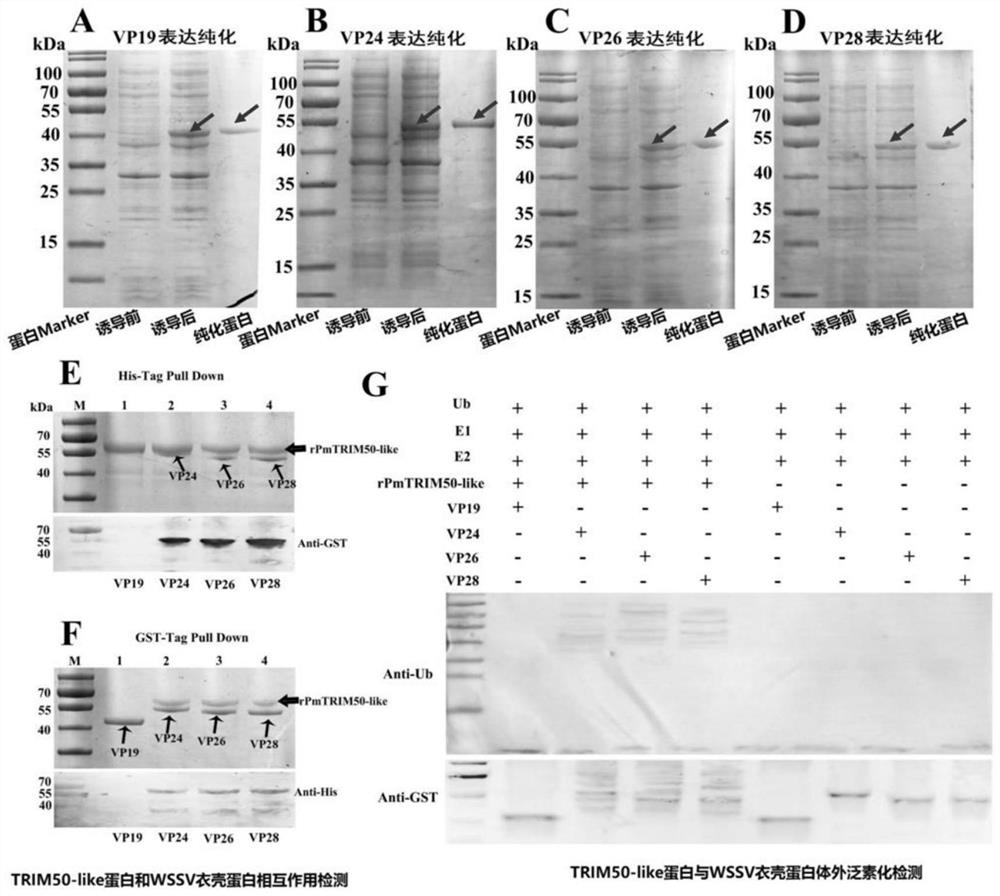
[0066] Example 3 Spotstine shrimp Trim50-LIKE protein with WSSV capsule protein interaction and in vitro ubiquitinization assay
[0067] (1) Spotstine shrimp Trim50-LIKE protein interact with WSSV capsule protein
[0068] First use the prokaryotic expression strain to express the purified WSSV vesicular protein VP19 (GenbankAccession No. DQ681071.1), VP24 (Genbank Accession No. DQ 199431.1), VP26 (GenbankAccession No. A220746.1) and VP28 (Genbank Accession) No.dq681069.1) figure 2 A-D Figure). For the specific implementation of the prokaryotic expression, as described in Example 1 (3), the original nuclear expression primer is shown below:
[0069] VP19:
[0070] RVP19-F GaattcatgcccaccacgactaacacacacAcCCCCCCCCCGACTAACAC
[0071] RVP19-R ctcgagttaatccctggtcctgttcttat
[0072] VP24:
[0073] RVP24-F GaattcatgcacatgtggggggTTTA
[0074] RVP24-R ctcgagtttttttcccccaaccttaa
[0075] VP26:
[0076] RVP26-F GGATCCACCCGTGTTGGAAGAAGCGT
[0077] RVP26-R GaattctTCTTCTTTTGATTCGTCCTTG
[0078] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com