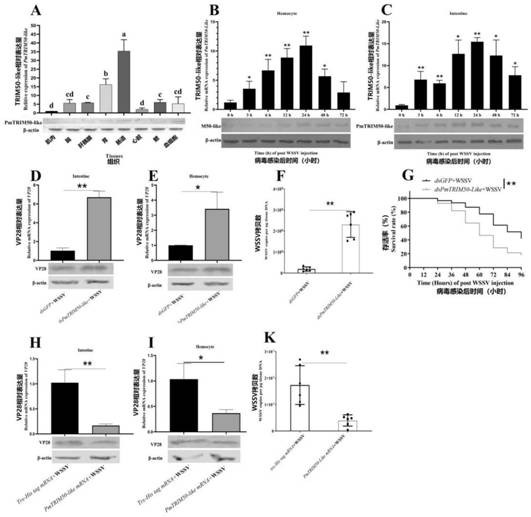
Penaeus monodon E3 ubiquitin ligase TRIM50-like protein and application thereof
A technology of ubiquitin ligase and Penaeus monodon, which is applied in the direction of ligase, peptide/protein components, enzymes, etc., to reduce the amount of replication and improve the survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Obtaining of Penaeus monodon E3 ubiquitin ligase TRIM50-like protein
[0035] (1) Extraction of total RNA
[0036] Take 50 mg of intestinal tissue of live and healthy Penaeus monodon, extract total RNA according to the instructions of the Qiagen Mini kit, and use DNase to digest and remove the genome.
[0037] (2) Preparation of full-length cDNA template
[0038] According to PrimeScript TM II 1st Strand cDNA Synthesis Kit (TaKaRa) manual to prepare full-length cDNA template, take 1 μg of total RNA, add 1 μL Oligo dT Primer (50 μM), 1 μL dNTP Mixture (10 mMeach), add RNase free ddH 2 O to make up to 10 μL. Mix the above solution, centrifuge briefly, and use a PCR instrument to keep the temperature at 65°C for 5 minutes, then take it out quickly, and cool it on ice to denature the template RNA. Add the following reagents sequentially: 4μL 5×PrimeScriptII Buffer, 0.5μL Inhibitor (40U / μL), 1μL PrimeScript II RTase (200U / μL) and RNasefree ddH 2 O. The follo...
Embodiment 2
[0063] Example 2 Preparation of TRIM50-like protein polyclonal antibody
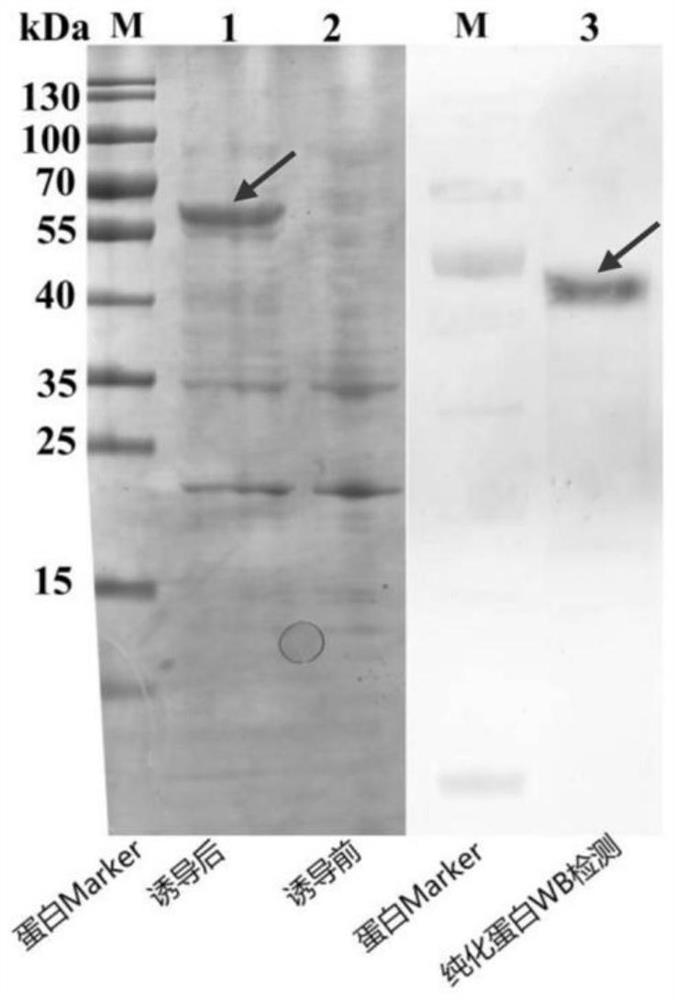
[0064] Mix the above-mentioned purified TRIM50-like recombinant protein (about 100-200 μg) with 3 mL of complete Freund’s adjuvant and incubate it, and inject it into mice subcutaneously at multiple points, and inject 8 mice in total, each mouse is injected with about 50 μg of protein . Three weeks after the first injection, three booster immunizations were given. The booster dose is 10-20 μg protein, emulsified with incomplete Freund's adjuvant. The interval between each injection is 10 days. After the 4th injection, the mouse eyeballs were removed to take blood. After standing still at 37°C for 1 hour, the blood was centrifuged at 4000rpm for 10 minutes.
[0065] Such as image 3 As shown, the polyclonal antibody prepared above for the TRIM50-like protein of Penaeus monodon can specifically bind to the endogenous TRIM50-like protein of Penaeus monodon, and different tissues of three healthy shrimps...
Embodiment 3
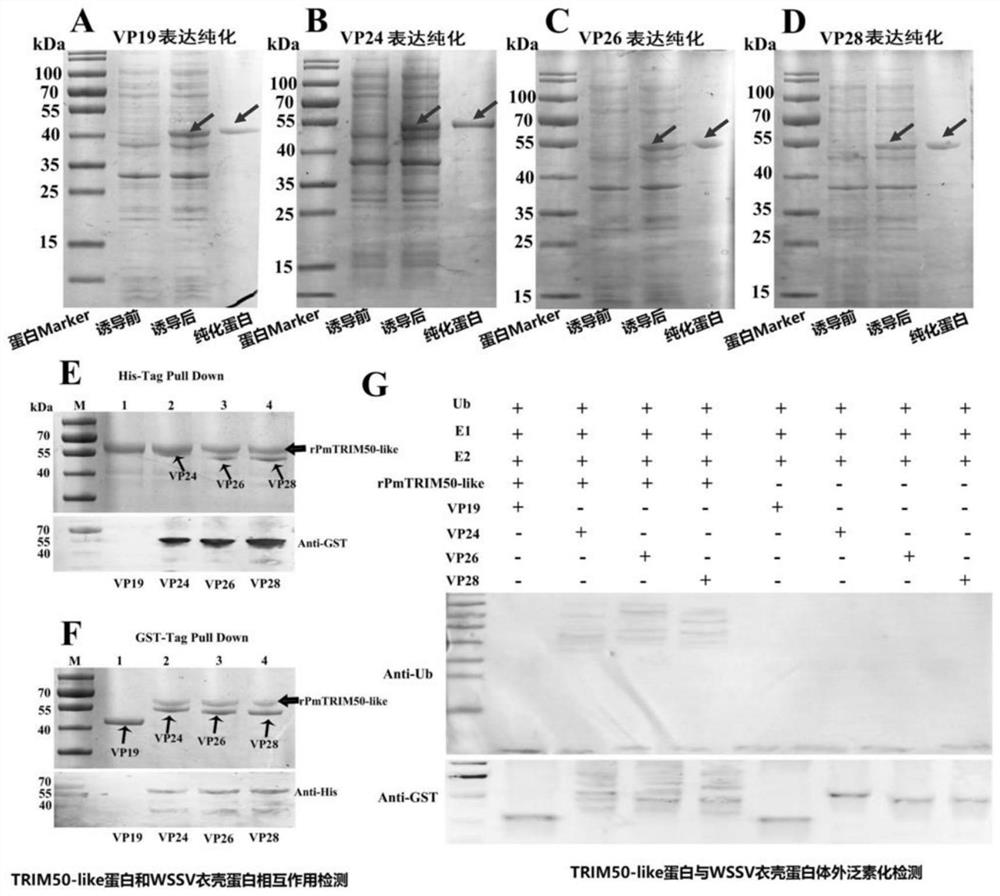
[0066] Example 3 Interaction between TRIM50-like protein of Penaeus monodon and WSSV envelope protein and in vitro ubiquitination assay
[0067] (1) Interaction between TRIM50-like protein of Penaeus monodon and WSSV envelope protein
[0068] First, the prokaryotic expression strains were used to express and purify WSSV envelope proteins VP19 (GenBank accession No.DQ681071.1), VP24 (GenBank accession No. DQ196431.1), VP26 (GenBank accession No. AY220746.1) and VP28 (GenBank accession No.DQ681069.1)( figure 2 middle panels A-D). The specific implementation method of prokaryotic expression is as described in "Example 1 (3)", and the prokaryotic expression primers are respectively as follows:
[0069] VP19:
[0070] rVP19-F GAATTCATGGCCACCACGACTAACAC
[0071] rVP19-R CTCGAGTTAATCCCTGGTCCTGTTCTTAT
[0072] VP24:
[0073] rVP24-F GAATTCATGCACATGTGGGGGGTTTA
[0074] rVP24-R CTCGAGTTATTTTTTCCCCAACCTTAA
[0075] VP26:
[0076] rVP26-F GGATCCACACGTGTTGGAAGAAGCGT
[0077] rVP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com