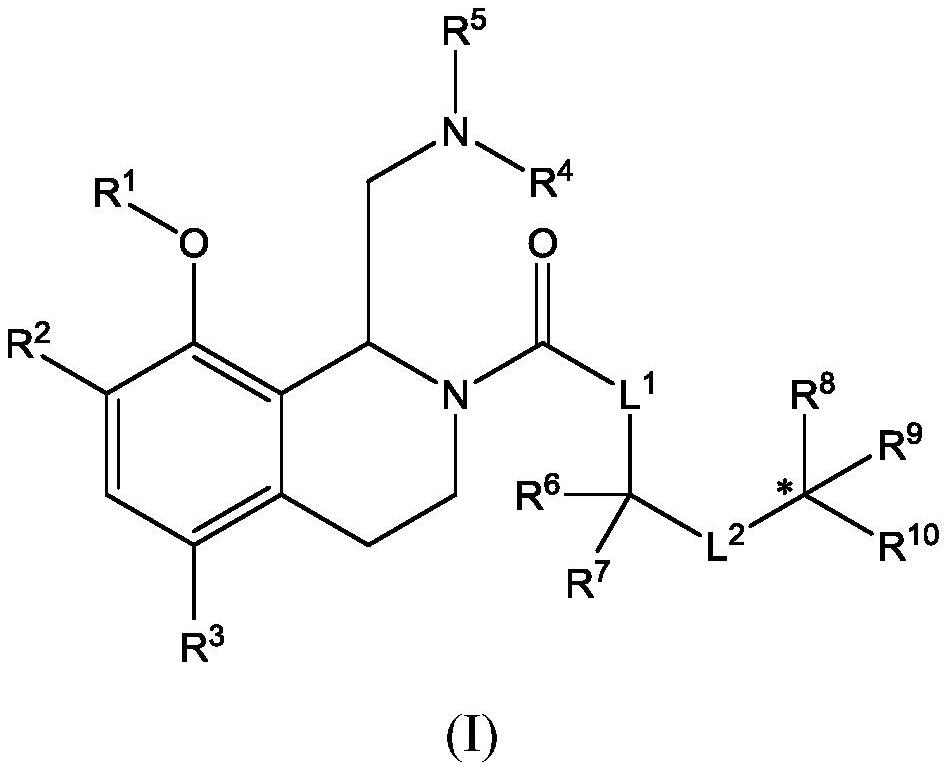
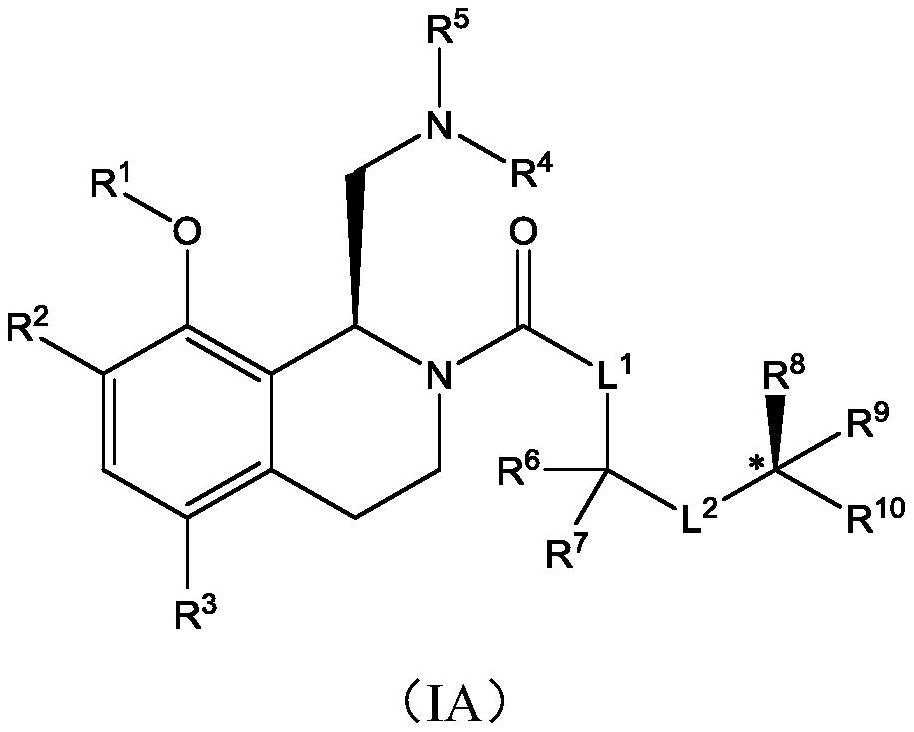
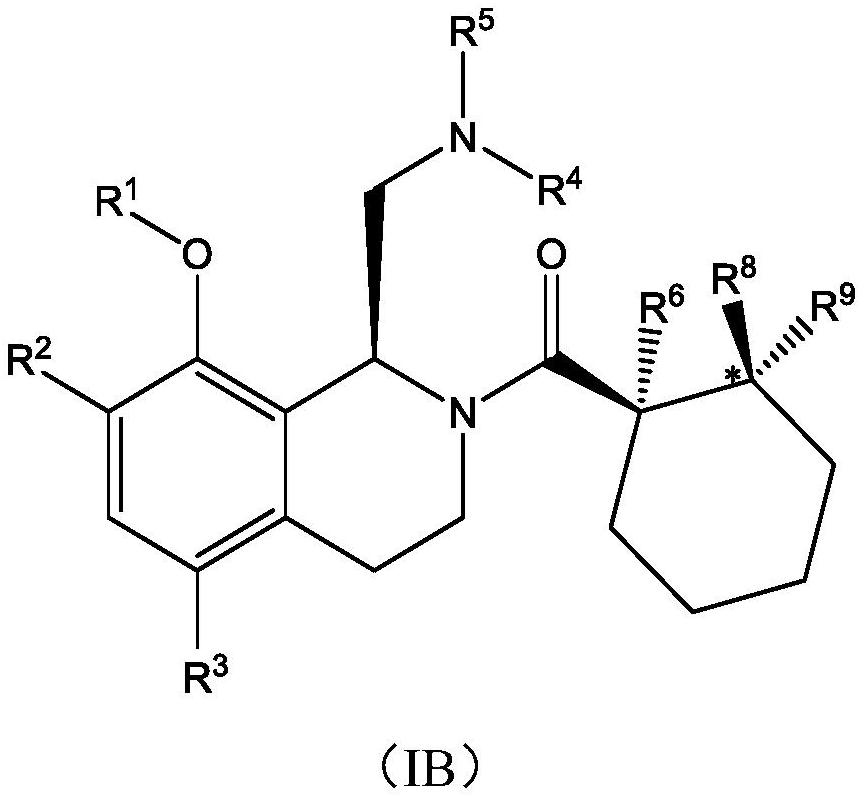
Therapeutic compounds
A technology selected from, alkyl, applied in the field of preparing these compounds, can solve the problem of patients prone to liver cirrhosis and liver-related complications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0763] General procedure:
[0764] Methods for preparing compounds of the present invention are shown in the examples below. Starting materials are prepared according to procedures known in the art or as shown herein, or are commercially available. Commercial reagents were used without further purification. The reaction is carried out at ambient temperature, typically 18°C to 27°C, excluding the reaction temperature.
[0765] The compounds described in the present invention are 1 In the case of H NMR spectral characterization, spectra were recorded on 500 MHz Bruker, 400 MHz Bruker, 250 MHz Bruker, 300 MHz JEOL, or 400 MHz JEOL instruments. Spectra were recorded at ambient temperature when temperature was not included. Chemical shift values are expressed in parts per million (ppm). In cases where NMR spectra are complicated by the presence of tautomers, approximate partial integration of the signal is reported, or only the characterization of the major isomer is rep...
example 1
[1091] Example 1: (1S,2R)-2-((S)-1-((1,3-dioxoisoindolin-2-yl)methyl)-8-(2-(5-methyl) different oxazole-3-carboxamide)ethoxy)-1,2,3,4-tetrahydroisoquinoline-2-carbonyl)cyclohexanecarboxylic acid
[1092]
[1093] Step a. To a stirred suspension of intermediate 11 (0.45 g, 1.1 mmol) and cesium carbonate (1.44 g, 4.4 mmol) in DMF (6 mL) at room temperature was added N-(2-chloroethyl)-bis Benzylamine hydrochloride (0.46 g, 1.5 mmol, CAS: 55-43-6) and the reaction was heated at 100 °C for 18 hours. The reaction mixture was concentrated in vacuo, diluted with water (25 mL), and extracted with DCM (25 mL). The organic layer was further washed with water (50 mL), washed with Na 2 SO 4 Dry and concentrate in vacuo to give tert-butyl (S)-8-(2-(dibenzylamino)ethoxy)-1-((1,3-dioxoisoindolin-2-yl) Methyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (1.10 g, assumed quantitative). 1 H NMR (400MHz, DMSO-d 6 , observed rotamers, all reported) δ 7.98-7.75 (m, 4H), 7.44-7.03 (m, 11H...
example 2
[1099] Example 2: (1S,2R)-2-((S)-8-(2-(benzo[d]oxazole-2-carboxamide)ethoxy)-1-((1,3-dioxo (isoindolin-2-yl)methyl)-1,2,3,4-tetrahydroisoquinoline-2-carbonyl)cyclohexane-1-carboxylic acid
[1100]
[1101] Step a. Benzyl(1S,2R)-2-(((S) was prepared from Intermediate 10 (350 mg, 1.14 mmol) and Intermediate 26 (351 mg, 1.25 mmol) using a procedure similar to that described in Example 1, Step e. )-1-((1,3-dioxoisoindolin-2-yl)methyl)-8-hydroxy-1,2,3,4-tetrahydroisoquinoline-2-carbonyl)cyclohexane Alkane-1-carboxylate (370 mg, 59%). LCMS (Method 4a): 2.86 min, 553.4 [M+H] + . 1H NMR (400MHz, DMSO-d 6) 9.86(br s, 1H), 7.86-7.79(m, 4H), 7.19-7.00(m, 5H), 6.73(d, 1H), 6.60(d, 1H), 5.98(dd, 1H), 4.77(q ,2H),4.22(dd,1H),3.80(d,1H),3.75-3.60(m,2H),3.29-3.24(m,1H),2.73-2.69(m,1H),2.40(dt,1H ), 1.84(q, 1H), 1.68-1.60(m, 1H), 1.50(d, 1H), 1.36-1.23(m, 3H), 1.00-0.76(m, 3H), 0.15(q, 1H).
[1102] Step b. Benzyl (1S,2R)-2-(((S)-) was prepared from the above intermediate and tert...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com