Preparation method of dalteparin sodium and application of method in preparation of low-molecular-weight heparin sodium
A technology of dalteparin sodium and molecular weight, which is applied in the field of dalteparin sodium preparation, can solve problems such as quality loss, and achieve the effects of reducing residue, convenient operation, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
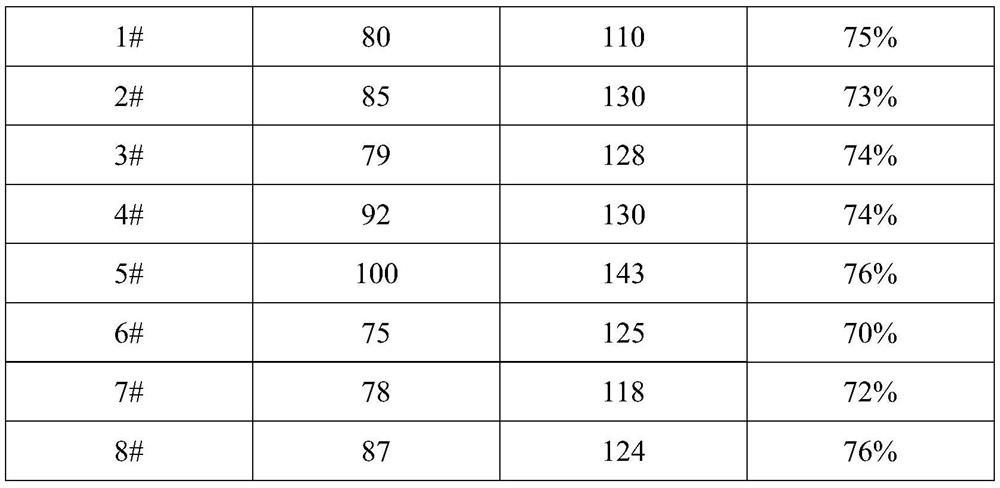
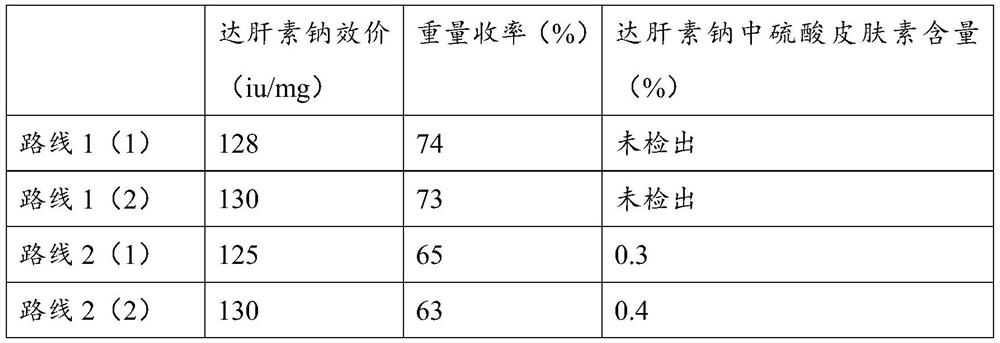
[0046] In this embodiment, a method for preparing dalteparin sodium using crude heparin sodium as a raw material is provided, and the preparation method includes the following steps:
[0047] (1) Crude product dissolution: Take crude heparin sodium, dissolve it at a concentration of 15% (w / v), add 3% (w / v) enteric salt, heat up to 52°C, and stir until dissolved.
[0048] (2) Primary enzymatic hydrolysis of the crude product: adjust the pH of the solution in (1) to 8.5 with 40% sodium hydroxide solution. Then add 0.5% trypsin by weight of crude heparin to start enzymatic hydrolysis. During the enzymolysis process, the pH was controlled within the range of 8.5-9.0, the temperature was controlled at 49°C, and the mixture was stirred every 20 minutes for 10 minutes each time at a speed of 60-70 rpm. The enzymolysis was performed for 5 hours, and the temperature and pH were recorded every 30 minutes.
[0049] (3) Heating up to remove impurity proteins: After enzymatic hydrolysis, ...
Embodiment 2
[0077] In the present embodiment, another preparation method of dalteparin sodium is provided, and the preparation method comprises the following steps:
[0078] (1) Dissolving the crude product: take the crude heparin sodium, dissolve it at a concentration of 14% (w / v), add 2% (w / v) enteric salt, heat up to 52°C, and stir until dissolved.
[0079] (2) Primary enzymatic hydrolysis of the crude product: adjust the pH of the solution in (1) to 9.0 with 40% sodium hydroxide solution. Then add 0.4% trypsin by weight of crude heparin to start enzymatic hydrolysis. During the enzymolysis process, control the pH within the range of 8.5-9.0, control the temperature at -52°C, stir once every 22 minutes, stir for 11 minutes each time, and rotate at 60-70rpm, enzymolysis for 4 hours, and record the temperature and pH every 30 minutes.
[0080] (3) Heating up to remove impurity proteins: After the enzymatic hydrolysis, adjust the pH to 6.0 with 4mol / L hydrochloric acid, quickly raise the...
Embodiment 3
[0108] (1) Crude product dissolution: take crude heparin sodium, dissolve it at a concentration of 16% (w / v), add 5% (w / v) enteric salt, heat up to 49°C, and stir until dissolved.
[0109] (2) Primary enzymatic hydrolysis of the crude product: adjust the pH of the solution in (1) to 9.0 with 40% sodium hydroxide solution. Then add 0.5% trypsin by weight of crude heparin to start enzymatic hydrolysis. During the enzymatic hydrolysis process, the pH was controlled within the range of 8.5-9.0, the temperature was controlled at 52°C, the mixture was stirred every 18 minutes, each stirring was 9 minutes, the speed was 60-70 rpm, the enzymatic hydrolysis was 6 hours, and the temperature and pH were recorded every 30 minutes.
[0110] (3) Heating up to remove impurity protein: After the enzymatic hydrolysis is completed, use 4mol / L hydrochloric acid to adjust the pH to 6.5±0.5, pass steam through the interlayer to quickly raise the temperature to 90°C, keep warm for 20min, then pass ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com