Aryl ketone compound as well as preparation method and application thereof
An aryl ketone and compound technology, applied in the field of aryl ketone compounds and their preparation, can solve the problems of low photoluminescence quantum yield and the like, and achieve the effects of improving photoluminescence quantum yield and high luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
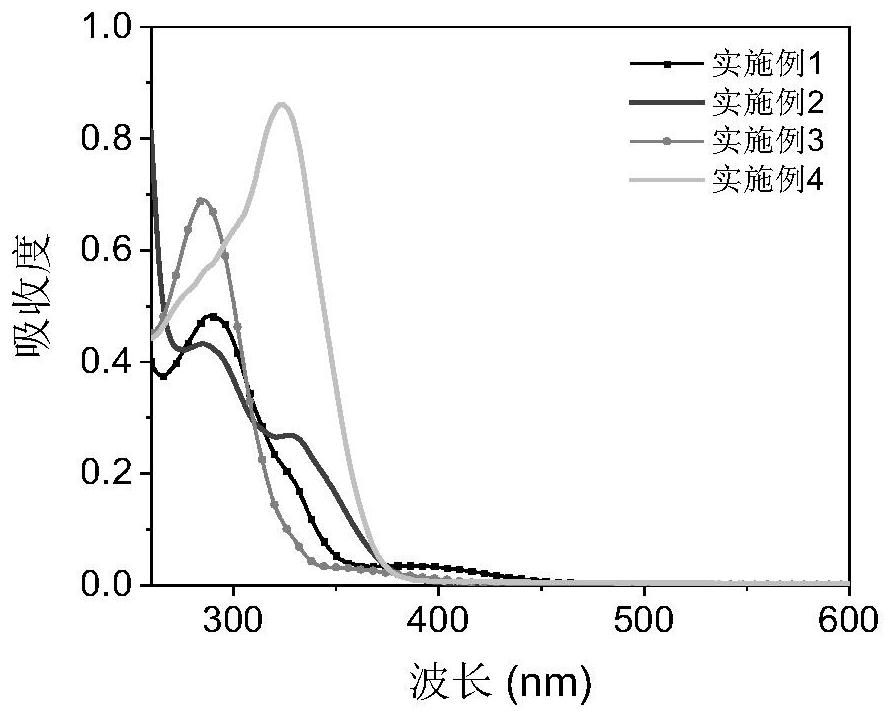
Embodiment 1
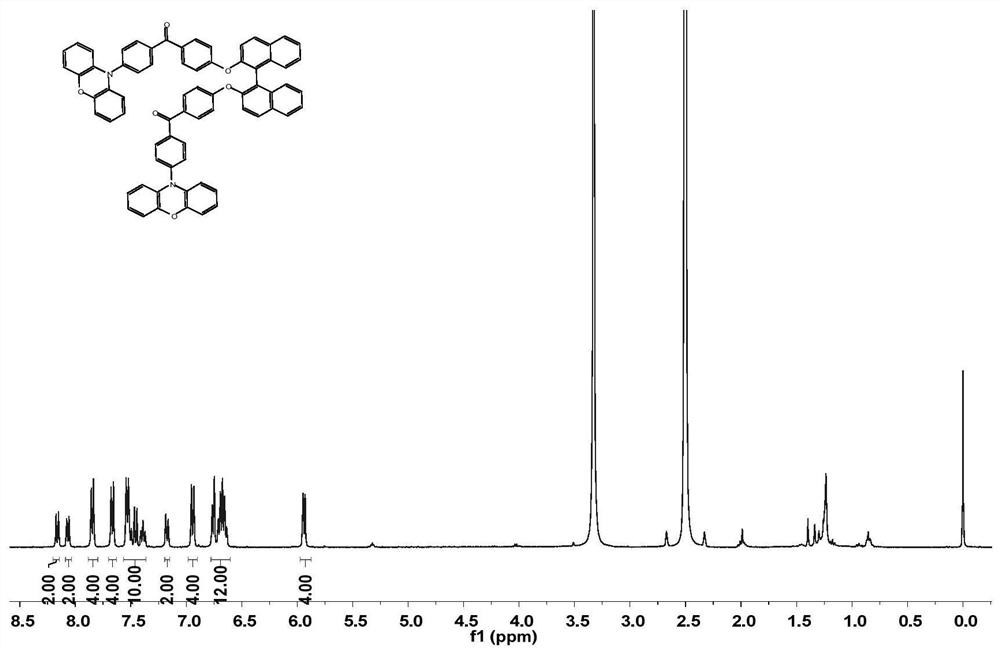
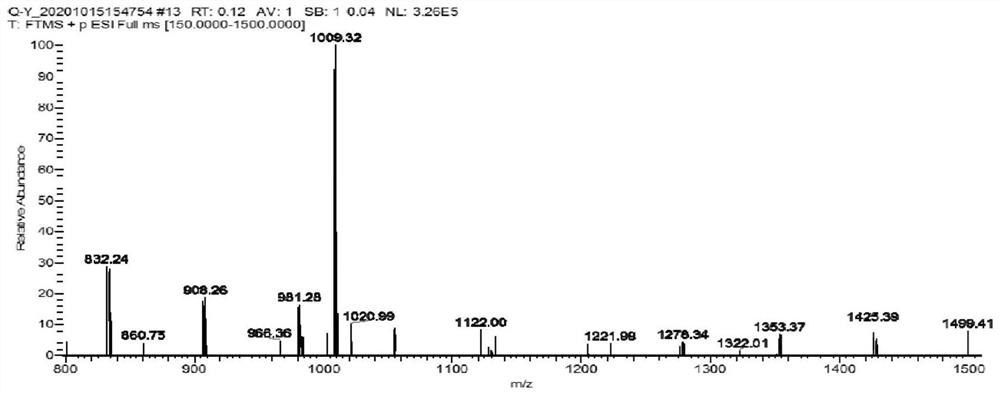
[0046] This example provides an aryl ketone compound, the structural formula of which is shown in A1,
[0047]
[0048] The preparation method of this compound is as follows:
[0049]
[0050] S1. Weigh binaphthol, cesium carbonate, 4,4-difluorobenzophenone and dissolve them in N,N-dimethylformamide in a 100mL round bottom flask at a molar ratio of 1:2:1. Under the condition of nitrogen protection, the above reactant was heated to 100° C. and reacted for 10 h. After the reaction was completed, the reaction liquid was cooled to room temperature, and extracted three times with ethyl acetate and saturated brine. The organic phase was taken, and the crude product was obtained after evaporating ethyl acetate under reduced pressure. Then, through silica gel column chromatography, using ethyl acetate / n-hexane as eluent, separation and purification are carried out to obtain intermediate 1, the structural formula of which is as follows
[0051]
[0052] S2. In a 100mL round...
Embodiment 2
[0054] This embodiment provides a kind of aryl ketone compound, its preparation Example 1 is the same, the difference is that, unlike Example 1, the groups of R1 and R2 are all. . The aryl ketone compound A2 is obtained, and the structural formula of A2 is shown below.
[0055]
Embodiment 3
[0057] This example provides an aryl ketone compound, the preparation method of which is the same as that of Example 1, the difference being that, unlike Example 1, the groups of R1 and R2 are The aryl ketone compound A3 is obtained, and the structural formula of A3 is shown below.
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com