Preparation method of lurasidone hydrochloride
A technology for lurasidone hydrochloride and hydrochloric acid solution, which is applied in organic chemistry and other fields, and can solve problems such as insoluble, product loss, and increased manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
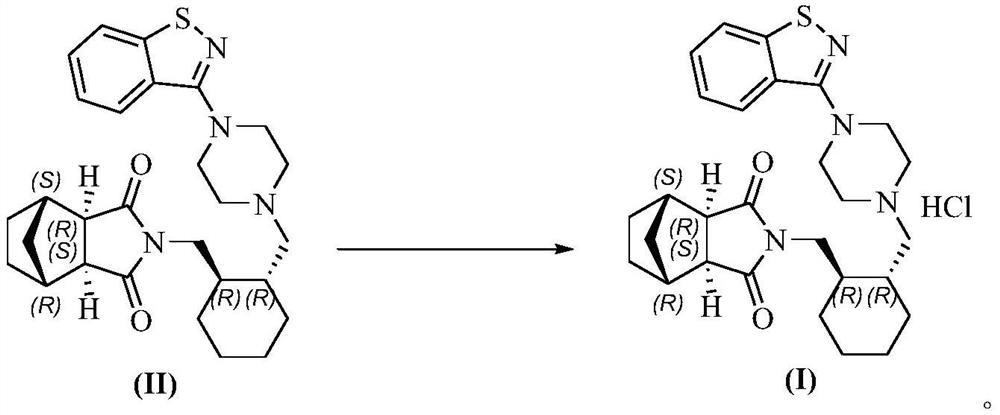
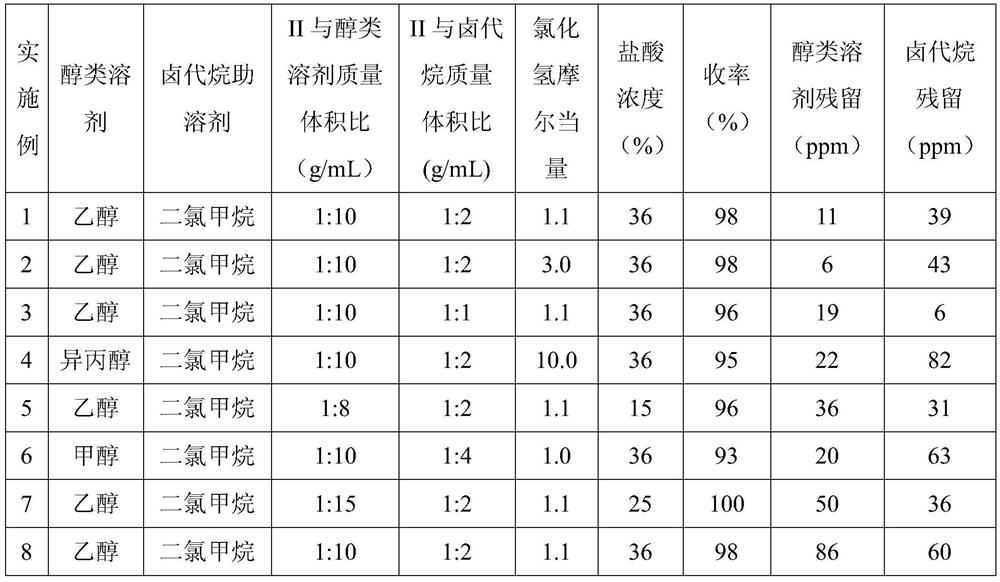
[0024] 10g of lurasidone free base (compound II), was added to the mixed system of 80mL ethanol and 20mL dichloromethane, the system was warmed up to reflux and dissolved, and then the premixed hydrochloric acid ethanol solution (2.26g 36% concentrated hydrochloric acid+ 20mL ethanol), after the dropwise addition, keep warm and reflux and stir for 1-2h, the system is turbid; start to cool down, within 2-3h, the system slowly cools down to 0-10°C, keep warm at 0-10°C, stir for 1-2h; filter, filter The cake was dried under vacuum at 45-50° C. to obtain 10.5 g of lurasidone hydrochloride (compound I), with a yield of 98%, residual ethanol: 11 ppm, and residual dichloromethane: 39 ppm.
Embodiment 2
[0026] 10g of lurasidone free base (compound II), was added to the mixed system of 90mL ethanol and 20mL dichloromethane, the system was warmed up to reflux and dissolved, and then the premixed hydrochloric acid ethanol solution (6.17g 36% concentrated hydrochloric acid+ 10mL ethanol), after the dropwise addition, keep warm and reflux and stir for 1-2h, the system is turbid; start to cool down, within 2-3h, the system slowly cools down to 0-10°C, keep warm at 0-10°C, stir for 1-2h; filter, filter The cake was dried under vacuum at 45-50° C. to obtain 10.5 g of lurasidone hydrochloride (compound I), with a yield of 98%, residual ethanol: 6 ppm, residual dichloromethane: 43 ppm.
Embodiment 3
[0028] Add 50g of lurasidone free base (compound II) into the mixed system of 500mL of ethanol and 50mL of dichloromethane, heat up the system to reflux and dissolve, then add 11.31g of 36% concentrated hydrochloric acid while keeping warm, after the addition is completed, keep stirring under reflux for 1~ 2h, the system was turbid; began to cool down, within 2-3h, the system slowly cooled down to 0-10°C, kept at 0-10°C, stirred for 1-2h; filtered, and the filter cake was vacuum-baked at 45-50°C to obtain 51.53g Lurasidone hydrochloride (compound I), yield 96%, residual ethanol: 19 ppm, residual dichloromethane: 6 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com