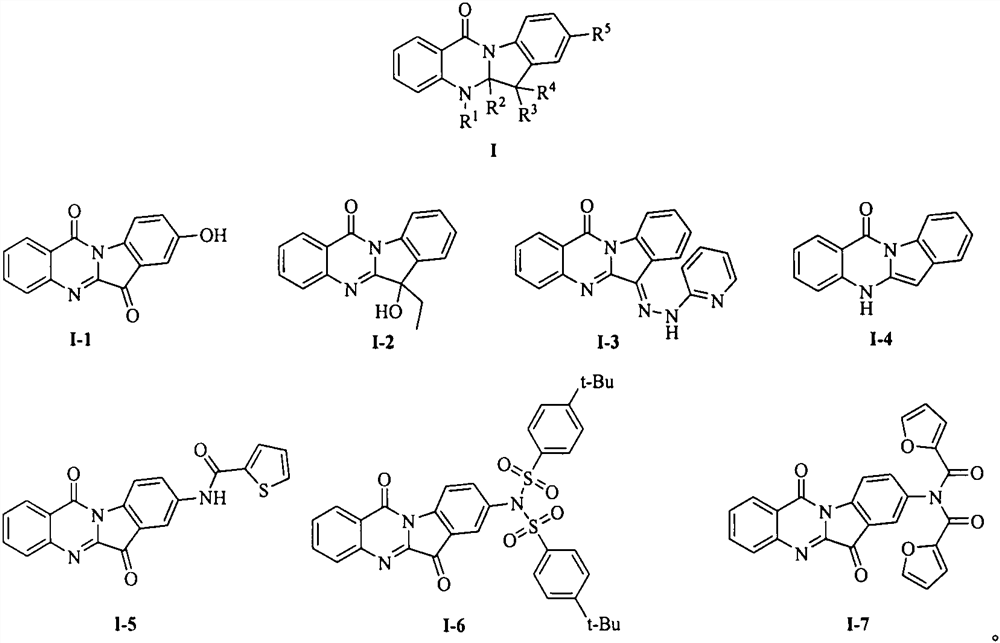
Tryptanthrin derivative and preparation thereof and application of tryptanthrin derivative in prevention and treatment of plant viruses and pathogenic bacteria
A technology of tryptanthrin and derivatives, applied in the fields of chemicals for biological control, botany equipment and methods, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
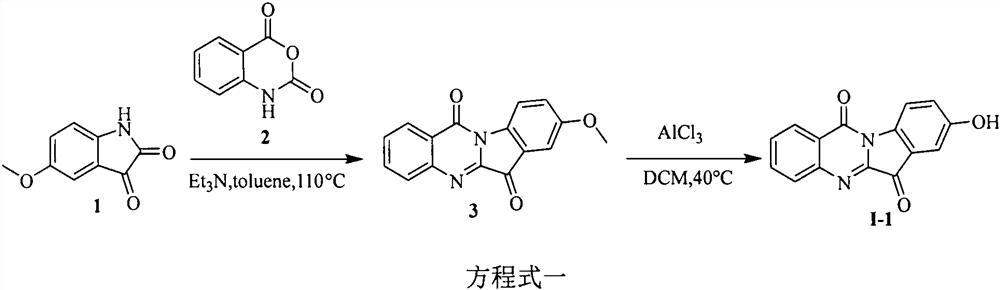
[0021] Embodiment 1: the synthesis of tryptanthrin derivative I-1
[0022] The first step, the synthesis of 8-methoxytryptanthrin (3). In a 100mL round bottom flask, add 5-methoxyisatin (1) (1.0g, 5.6mmol), isatoic anhydride (2) (0.92g, 5.6mmol), triethylamine (4.0mL, 28.0mmol) and toluene (40mL), heated to reflux at 110°C for six hours, TLC monitored the completion of the reaction, and suction filtered to obtain a yellow solid (product), the filtrate was diluted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, and suction filtered. Precipitation under reduced pressure, column chromatography (V (petroleum ether): V (ethyl acetate) = 1: 1), a yellow solid was obtained, and the products were combined to obtain a total of 1.1 g of the product, with a yield of 73% and a melting point of 270-272°C . 1 H NMR (400MHz, DMSO-d6) δ8.37(d, J=8.5Hz, 1H), 8.30(d, J=7.9Hz, 1H), 7.93(d, J=3.7Hz, 2H), 7.73(dt , J=8.2, 4.2Hz, 1H), 7.45-7.39(m, 2H), 3.87(...
Embodiment 2
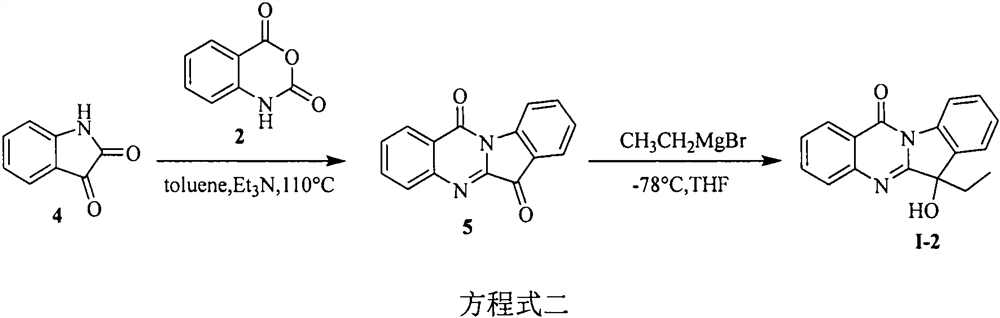
[0024] Example 2: Synthesis of tryptanthrin derivative I-2.
[0025] The first step: the synthesis of tryptanthrin (5). In a 100 mL round bottom flask was added isatin (4) (1.0 g, 6.8 mmol), isatoic anhydride (2) (1.12 g, 6.8 mmol), triethylamine (4.8 mL, 34 mmol) and toluene (40 mL) , heated to reflux at 110°C for six hours, TLC monitored that the reaction was complete, suction filtered to obtain a yellow solid (product), the filtrate was diluted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, suction filtered, and precipitated under reduced pressure, Column chromatography (V (petroleum ether): V (ethyl acetate) = 1: 1) gave a yellow solid. The products were combined to obtain 1.6 g of the product in a yield of 95%. The melting point was 267-269°C. 1 H NMR (400MHz, DMSO-d6) δ8.47(d, J=7.9Hz, 1H), 8.31(d, J=7.8Hz, 1H), 7.94(d, J=3.8Hz, 2H), 7.91-7.84 (m, 2H), 7.78-7.69(m, 1H), 7.48(t, J=7.5Hz, 1H). 13 C NMR (100MHz, DMSO-d6) δ 182.4, 15...
Embodiment 3
[0027] Example 3: Synthesis of tryptanthrin derivative I-3.
[0028] Dissolve tryptanthrin (5) (1.0 g, 4.0 mmol) in 50 mL of anhydrous methanol, gradually add 2-hydrazinopyridine (6) (0.66 g, 6.0 mmol), and heat to reflux at 70°C for 6 h under argon protection. After the reaction was completed, it was cooled to room temperature, suction filtered, and the filter cake was washed with methanol to obtain 0.7 g of a red solid with a yield of 52% and a melting point of 214-216°C. 1 H NMR (400MHz, CDCl 3 )δ13.40(s, 1H), 8.59(d, J=8.0Hz, 1H), 8.46(d, J=7.7Hz, 1H), 8.36(d, J=4.5Hz, 1H), 7.99(d, J=8.1Hz, 1H), 7.90(d, J=7.5Hz, 1H), 7.82(t, J=7.6Hz, 1H), 7.78-7.73(m, 1H), 7.72-7.68(m, 1H), 7.58(t, J=7.5Hz, 1H), 7.48(t, J=7.7Hz, 1H), 7.39(t, J=7.5Hz, 1H), 7.01(t, 1H). 13 C NMR (100MHz, CDCl 3C 20 h 13 N 5 O[M+H] + 340.1193, found 340.1195.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com