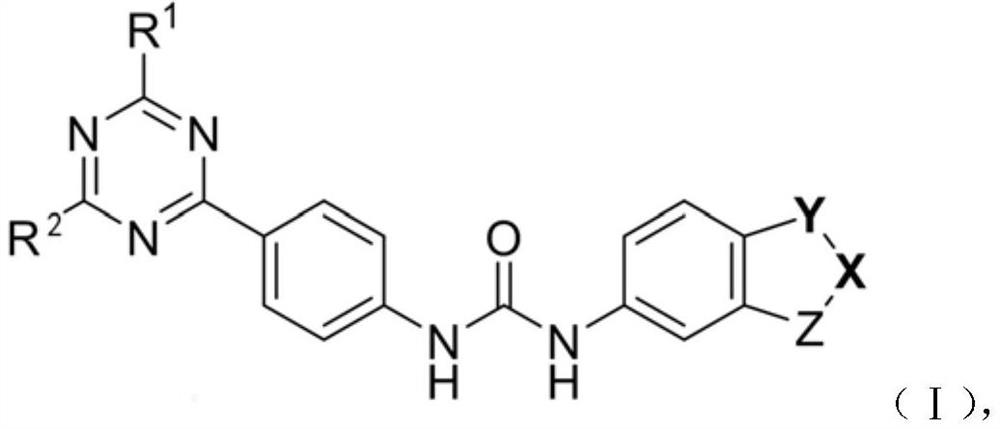
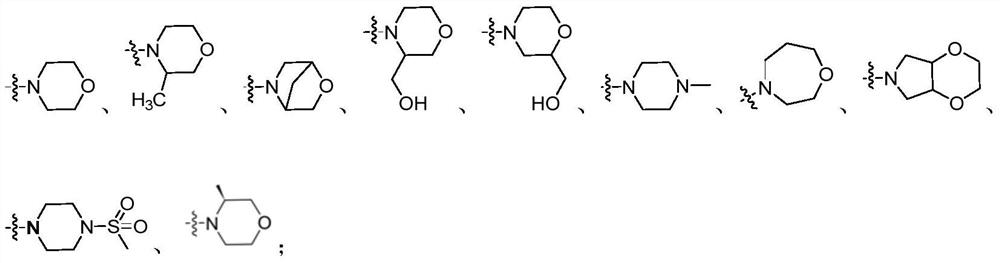
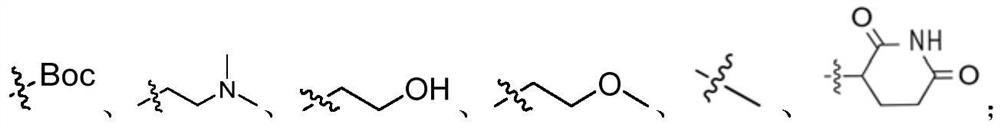
Diarylurea PI3Kalpha/mTOR double-target inhibitor and pharmaceutical composition, and application of inhibitor and pharmaceutical composition
A diarylurea, dual-target technology, applied in the field of medicine, can solve problems such as drug resistance of single mTOR inhibitors, and achieve the effects of significant anti-tumor activity, novel structure and good development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1: 6-(3-(4-(4,6-dimorpholine-1,3,5-triazin-2-yl)phenyl)ureido)-1-oxoisoindoline- tert-Butyl 2-carboxylate
[0144] Step 1: Synthesis of 4,4'-(6-chloro-1,3,5-triazine-2,4-diyl)dimorpholine
[0145] The structural formula of 4,4'-(6-chloro-1,3,5-triazine-2,4-diyl)dimorpholine:
[0146] Synthetic method: add cyanuric chloride (0.5g, 2.7mmol) in 50mL reaction flask, with anhydrous CH 2 Cl 2 Dissolve, cool to -10°C, slowly add a mixture of morpholine (0.47g, 5.4mmol) and triethylamine (0.5ml, 2.7mmol) dropwise, after the addition is complete, move the reaction to 0°C for 1h, then rise to room temperature Reaction 3h. A white solid precipitated out of the reaction solution. After TLC detection reaction finishes, reaction solution is poured in the ice water (50ml), separates CH 2 Cl 2 layer, the aqueous phase with CH 2 Cl 2 (100mL) was extracted once. The combined organic layers were washed once with saturated NaCl aqueous solution (50 mL), and the organic ...
Embodiment 2
[0153] Example 2: 1-(4-(4,6-dimorpholin-1,3,5-triazin-2-yl)phenyl)-3-(3-oxoisoindoline-5-yl ) urea
[0154] The structural formula of 1-(4-(4,6-dimorpholin-1,3,5-triazin-2-yl)phenyl)-3-(3-oxoisoindoline-5-yl)urea :
[0155] Synthesis method: 6-(3-(4-(4,6-dimorpholine-1,3,5-triazin-2-yl)phenyl)ureido)-1-oxo in Example 1 Dissolve tert-butyl isoindoline-2-carboxylate (0.05g, 0.08mmol) in redistilled DCM (2ml), cool to 0°C, slowly add trifluoroacetic acid (0.018ml) dropwise, move to room temperature and stir 2h. TLC monitored the completion of the reaction. The solvent was removed by evaporation to obtain a crude product, which was then purified by silica gel column chromatography (DCM / MeOH=10 / 1) to obtain 30 mg of the target compound, yield: 72%. HRMS(ESI)calcd.for C26H29N8O4[M+H] + :517.2312,found:517.2308. The NMR data are 1 H NMR (400MHz, DMSO-d 6 )δ: 9.21(s, 2H), 8.55(s, 1H), 8.28(d, J=8.8Hz, 2H), 7.91(d, J=1.9Hz, 1H), 7.62–7.54(m, 3H), 7.47 (d, J = 8.2Hz, 1H), 4....
Embodiment 3
[0156] Example 3: 5-(3-(4-(4,6-dimorpholine-1,3,5-triazin-2-yl)phenyl)ureido)-1,3-dioxaindole tert-Butyl-line-2-carboxylate
[0157] 5-(3-(4-(4,6-Dimorpholine-1,3,5-triazin-2-yl)phenyl)ureido)-1,3-dioxaindoline-2- The structural formula of tert-butyl carboxylate:
[0158] Synthetic method: Change the condensed heterocyclic segment in Step 3 of Example 1 to tert-butyl 5-amino-1,3-dioxaindoline-2-carboxylate, and other steps and operations are similar to Example 1. Yield: 65.6%. HRMS(ESI)calcd.forC31H35N8O7[M+H] + :631.2629,found:631.2627. The NMR data are 1 H NMR (400MHz, DMSO-d 6 )δ: 9.64(s, 1H), 9.21(s, 1H), 7.92–7.88(m, 2H), 7.77(d, J=1.9Hz, 1H), 7.46(d, J=8.3Hz, 1H), 7.36(dd,J=8.3,1.9Hz,1H),7.21–7.17(m,2H),3.43(d,J=34.3Hz,8H),3.28–3.24(m,8H),1.15(s,9H) . 13 C NMR (100MHz, DMSO-d 6 )δ: 165.96, 165.71, 164.14, 164.06, 152.77, 148.11, 138.76, 138.70, 135.89, 131.14, 130.89, 129.77, 123.41, 121.02, 120.53, 114.90, 83.49, 456.89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com