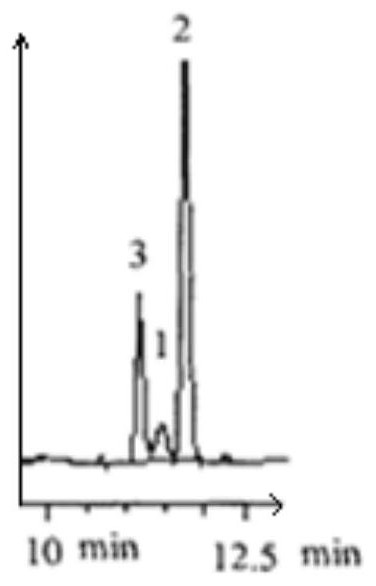
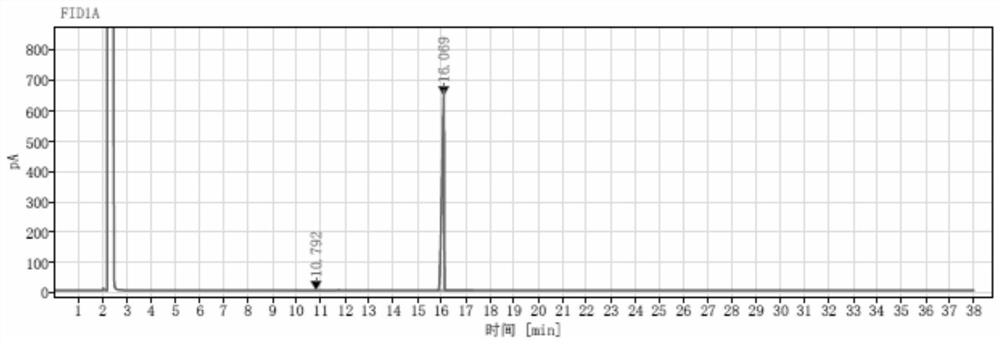
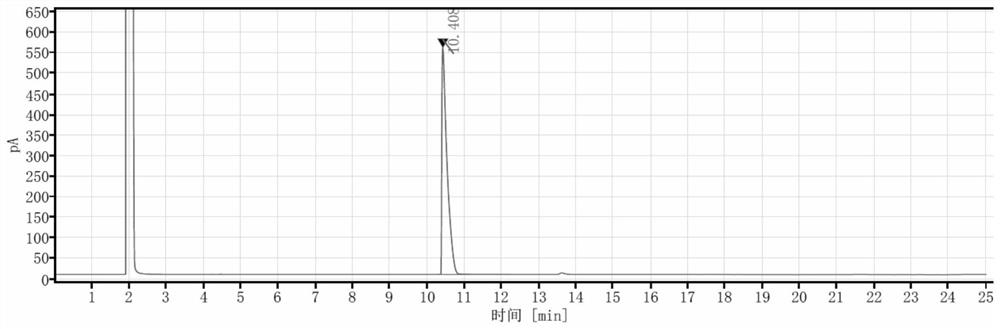
Production process and application of (2S, 5S)-2, 5-hexanediol
A production process and technology of hexylene glycol, applied in the field of bioengineering, can solve the problem of low product conversion rate, and achieve the effects of simple reaction operation and broad market prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A production process of (2S,5S)-2,5-hexanediol, specifically comprising the following steps:
[0042] With 2,5-hexanedione as the initial substrate of the reaction, with ketoreductase (the ketoreductase is a reductase encoded by the ketoreductase gene, and the amino acid sequence of the ketoreductase is as shown in SEQ ID No.2 Shown, the nucleotide sequence of the ketoreductase gene is shown in SEQ ID No.1) as a catalyst, adding coenzyme and isopropanol to form a reaction system for catalytic reaction, to obtain the target product, that is, the (2S, 5S) -2,5-Hexanediol. Specifically, the concentration of the initial substrate 2,5-hexanedione was controlled at 877mol L -1 About, the concentration of ketoreductase in described reaction system is 50g / L, the concentration of coenzyme NAD in described reaction system is 0.1g / L, add and account for 80% v / v of the total volume of described reaction system Propanol, remove the acetone generated by vacuuming in the reaction, a...
Embodiment 2
[0044] A production process of (2S,5S)-2,5-hexanediol, specifically comprising the following steps:
[0045] With 2,5-hexanedione as the initial substrate of the reaction, with ketoreductase (the ketoreductase is a reductase encoded by the ketoreductase gene, and the amino acid sequence of the ketoreductase is as shown in SEQ ID No.2 Shown, the nucleotide sequence of the ketoreductase gene is shown in SEQ ID No.1) as a catalyst, adding coenzyme and isopropanol to form a reaction system for catalytic reaction, to obtain the target product, that is, the (2S, 5S) -2,5-Hexanediol. Specifically, the concentration of the initial substrate 2,5-hexanedione is controlled at 800mol L -1 About, the concentration of ketoreductase in the reaction system is 40g / L, the concentration of coenzyme NAD in the reaction system is 0.01g / L, and 70% v / v of the total volume of the reaction system is added Propanol, remove the generated acetone by vacuuming during the reaction, add isopropanol to pro...
Embodiment 3
[0047] A production process of (2S,5S)-2,5-hexanediol, specifically comprising the following steps:
[0048] With 2,5-hexanedione as the initial substrate of the reaction, with ketoreductase (the ketoreductase is a reductase encoded by the ketoreductase gene, and the amino acid sequence of the ketoreductase is as shown in SEQ ID No.2 Shown, the nucleotide sequence of the ketoreductase gene is shown in SEQ ID No.1) as a catalyst, adding coenzyme and isopropanol to form a reaction system for catalytic reaction, to obtain the target product, that is, the (2S, 5S) -2,5-Hexanediol. Specifically, the concentration of the initial substrate 2,5-hexanedione is controlled at 900mol L -1 Left and right, the concentration of ketoreductase in the reaction system is 60g / L, the concentration of coenzyme NAD in the reaction system is 1g / L, and isopropyl Alcohol, remove the generated acetone by vacuuming during the reaction, add isopropanol to promote the reaction, and the catalytic reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com