Method for preparing acyl-L-carnitine and/or L-carnitine
A technology of carnitine and acyl, which is applied in the field of preparing acyl-L-carnitine and/or L-carnitine, and can solve the problems of few types of acyl-L-carnitine products, inability to recycle crotonbetaine, and the conversion rate of precursors low level problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
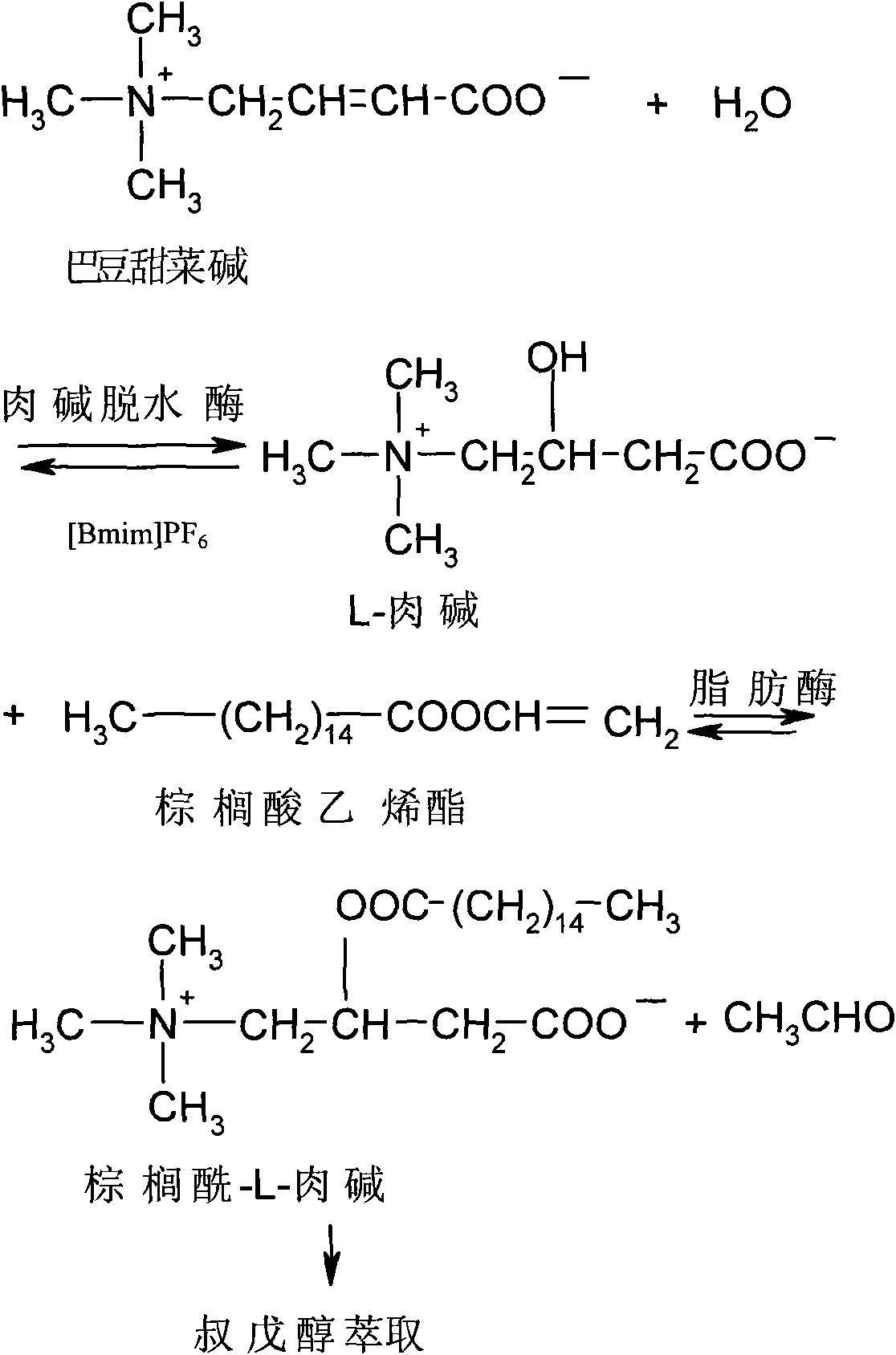
[0032] Embodiment 1, preparation palmitoyl-L-carnitine
[0033] 1) Mix 20ml of [Bmim]PF 6 Ionic liquid (purchased from Henan Lihua Pharmaceutical Co., Ltd.), 1.5mmol croton betaine (Fan Liqiang, Yuan Qinsheng. Microbial conversion method-a new idea of DL-carnitine untwisting [J]. Medicinal Chemistry, 1999, 34 (5): 335-337), 100mg KNO 3 , 200 mg of carnitine dehydratase and 1 ml of pH6.5 phosphate buffer solution were placed in a 125 ml round bottom flask, and reacted for 3 hours at 40° C. and 120 rpm on a vacuum rotary evaporator to obtain L-carnitine.
[0034] The reaction at this stage is the hydration reaction of L-carnitine from croton betaine catalyzed by carnitine dehydratase.
[0035] 2) Centrifuge the reaction solution of the above step 1) at 4200rpm for 1min to remove the carnitine dehydratase in the reaction system, place the separated carnitine dehydratase in 1ml of pH 6.5 phosphate buffer solution for the hydration reaction of the next cycle ; Then keep the re...
Embodiment 2
[0043] Embodiment 2, preparation palmitoyl-L-carnitine (the process of step 2-4 is not repeated)
[0044] 1) Mix 20ml of [Bmim]PF 6 Ionic liquid (purchased from Henan Lihua Pharmaceutical Co., Ltd.), 1.5mmol croton betaine (Fan Liqiang, Yuan Qinsheng. Microbial conversion method-a new idea of DL-carnitine untwisting [J]. Medicinal Chemistry, 1999, 34 (5): 335-337), 100mg KNO 3 , 200mg of carnitine dehydratase and 1ml of pH6.5 phosphate buffer solution were placed in a 125ml round bottom flask, and reacted for 12h on a vacuum rotary evaporator at 30°C and 120rpm to obtain L-carnitine.
[0045]The reaction at this stage is the hydration reaction of L-carnitine from croton betaine catalyzed by carnitine dehydratase.
[0046] 2) Centrifuge the reaction solution in the above step 1) at 500rpm for 30min to remove the carnitine dehydratase in the reaction system, place the separated carnitine dehydratase in 1ml of pH 6.5 phosphate buffer solution for the hydration reaction of the...
Embodiment 3
[0052] Embodiment 3, preparation of palmitoyl-L-carnitine (the process of steps 2-4 is repeated once)
[0053] 1) Mix 20ml of [Bmim]PF 6 Ionic liquid (purchased from Henan Lihua Pharmaceutical Co., Ltd.), 1.5mmol croton betaine (Fan Liqiang, Yuan Qinsheng. Microbial conversion method-a new idea of DL-carnitine untwisting [J]. Medicinal Chemistry, 1999, 34 (5): 335-337), 50mg fumaric acid, 200mg carnitine dehydratase and 1ml pH6.5 phosphate buffer solution were placed in a 125ml round bottom flask, and reacted for 2h at 45°C and 120rpm on a vacuum rotary evaporator to obtain L- carnitine.
[0054] The reaction at this stage is the hydration reaction of L-carnitine from croton betaine catalyzed by carnitine dehydratase.
[0055] 2) Centrifuge the reaction solution of the above step 1) at 10000rpm for 0.5min to remove the carnitine dehydratase in the reaction system, place the separated carnitine dehydratase in 1ml of pH 6.5 phosphate buffer solution for the next cycle of hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com