A polypeptide that selectively degrades pd-l1 protein on tumor cell membrane and its application
A selective and biologically active technology, applied in the field of peptides and applications that selectively degrade PD-L1 protein on tumor cell membranes, to achieve high biocompatibility, inhibit growth speed, and restore immune killing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
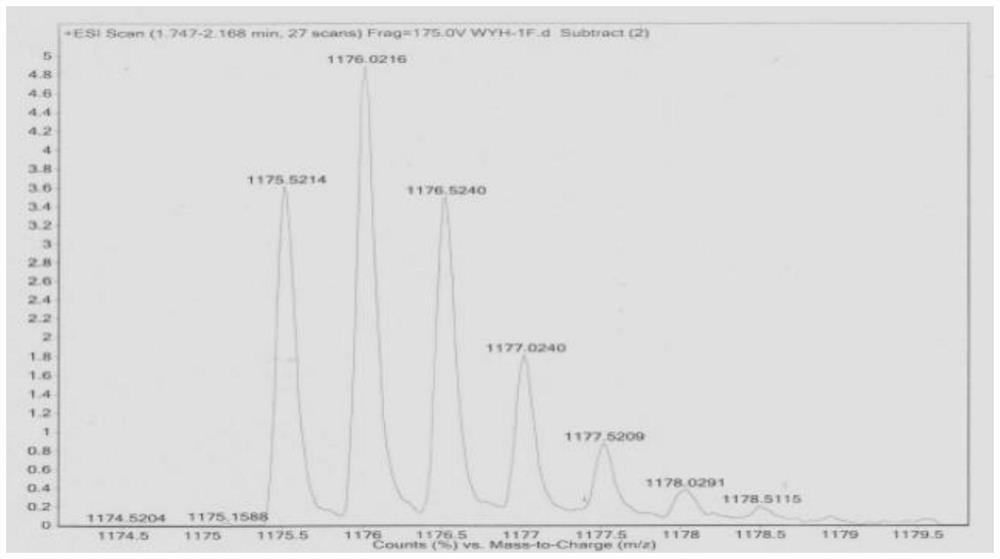
[0072] Example 1 Polypeptide Ada-GG D F D F D pYG D N D Y D S D K D P D T D D D R D Q D Y D H D Synthesis of F
[0073] It was synthesized by Fmoc-short peptide solid-phase synthesis method. The specific steps are:
[0074] 1) Weigh 0.5 mmol of 2-Cl-Trt resin in a solid phase synthesizer, add 10 mL of anhydrous dichloromethane (DCM), place it on a shaking table and shake for 10 min to fully swell the 2-Cl-Trt resin;
[0075] 2) Use ear washing balls to remove DCM from the solid-phase synthesizer equipped with 2-Cl-Trt resin;
[0076] 3) Dissolve 0.5 mmol of Fmoc-protected amino acid (Fmoc-D-Phe-OH) in 10 mL of anhydrous DCM, add 1 mmol of DIEPA, then transfer to the above solid-phase synthesizer, and react at room temperature for 1 h;
[0077] 4) Closure: remove the reaction solution in the solid-phase synthesizer with ear washing balls, then wash with 10 mL of anhydrous DCM for 1 min each, for a total of 5 washes, add the prepared volume ratio of anhydrous DC...
Embodiment 2
[0083] Example 2 Polypeptide Ada-GGGG D pYG D N D Y D S D K D P D T D D D R D Q D Y D H D Synthesis of F
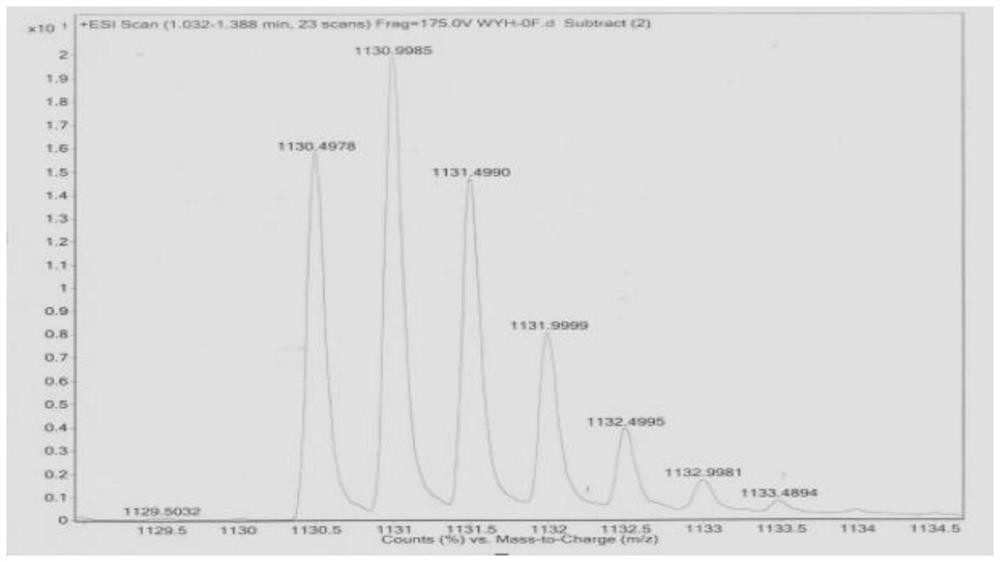
[0084] Polypeptides without phenylalanine were synthesized according to the solid-phase synthesis method of Fmoc-short peptide in Example 1. The obtained polypeptide is referred to as OF, and its amino acid sequence is shown in SEQ ID NO.2. The polypeptide obtained in Example 2 was detected by high-performance liquid chromatography-mass spectrometry, and the results were shown in figure 2 , the structural formula is shown in claim 3.
Embodiment 3
[0085] Example 3 Polypeptide Ada-GGG D F D pYG D N D Y D S D K D P D T D D D R D Q D Y D H D Synthesis of F
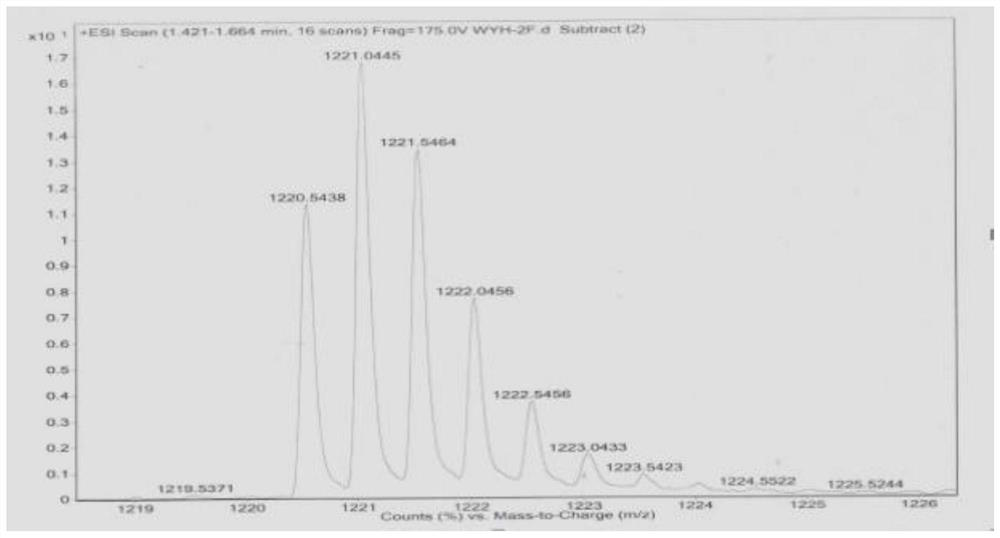
[0086] A polypeptide containing one phenylalanine was synthesized according to the solid-phase synthesis method of Fmoc-short peptide in Example 1. The obtained polypeptide is referred to as 1F for short, and the amino acid sequence is shown in SEQ ID NO.3. The polypeptide obtained in Example 3 was detected by high-performance liquid chromatography-mass spectrometry, and the results were shown in image 3 , the structural formula is shown in claim 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com