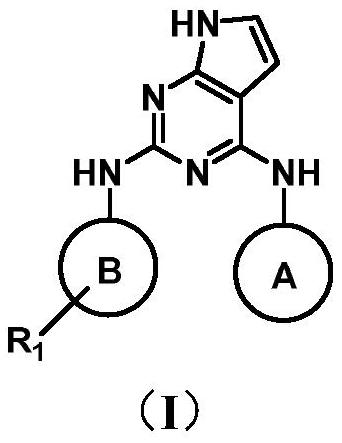
Pyrrolopyrimidine derivative as well as preparation method and application thereof
A technology of compounds and hydrates, applied in chemical instruments and methods, drug combinations, pharmaceutical formulations, etc., can solve problems such as no clinical research reports, and achieve good inhibition, good inhibition effect, and good cell proliferation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: N-ethyl-4-((4-((1-methyl-2-oxo-1,2-dihydropyridin-3-yl)amino)-7H-pyrrole[2,3-d ]pyrimidin-2-yl)amino)benzenesulfonamide
[0098]
[0099] 1 H NMR (600MHz, DMSO-d 6 )δ11.52(s,1H),9.54(s,1H),8.71(dd,J=7.4,1.5Hz,1H),8.38(s,1H),8.01(d,J=8.9Hz,2H), 7.65(d, J=8.9Hz, 2H), 7.40(dd, J=6.8, 1.7Hz, 1H), 7.30(t, J=5.8Hz, 1H), 7.06(dd, J=3.4, 2.3Hz, 1H ),6.56(dd,J=3.4,1.9Hz,1H),6.32(t,J=7.1Hz,1H),3.58(s,3H),2.76(dt,J=13.1,6.5Hz,2H),0.97 (t,J=7.2Hz,3H).ESI-MS m / z:440.1[M+H] + .
[0100] synthetic route
[0101]
[0102] Reagents and conditions: a) CH 3 I, NaH, anhydrous THF, 55°C; b) Pd / C, H 2 , MeOH, 40℃; c) Ts-Cl, NaH, anhydrous THF, 0℃; d) 1-3, DIPEA, DMF, 100℃; e) Pd(AcO) 2 ,X-phos,Cs 2 CO 3 , dioxane, 90°C; f) NaOH, MeOH / H 2 O, 40°C.
[0103] a) Dissolve 3-nitropyridin-2(1H)-one (14.2mmol) in 30mL of anhydrous tetrahydrofuran, add sodium hydride (18.5mmol) in batches under stirring at 0°C, and react for 0.5h. Then iodomethane (16.9 mmol) was added ...
Embodiment 2
[0110] Example 2: N-(4-((4-((4-((1-methyl-2-oxo-1,2-dihydropyridin-3-yl)amino)-7H-pyrrole[2,3 -d]pyrimidin-2-yl)amino)phenyl)acetamide
[0111]
[0112] 1 H NMR (600MHz, DMSO-d 6 )δ11.34(s,1H),11.34(s,1H),9.77(s,1H),9.77(s,1H),8.94(s,1H),8.94(s,1H),8.74(d,J =6.6Hz,1H),8.74(d,J=6.6Hz,1H),8.25(s,1H),8.25(s,1H),7.70(d,J=8.9Hz,2H),7.70(d,J =8.9Hz,2H),7.45(d,J=8.9Hz,2H),7.45(d,J=8.9Hz,2H),7.35(dd,J=6.8,1.7Hz,1H),7.35(dd,J =6.8,1.7Hz,1H),6.97(dd,J=3.4,2.3Hz,1H),6.48(dd,J=3.4,1.9Hz,1H),6.29(t,J=7.1Hz,1H),3.57 (s,3H),2.02(s,3H).ESI-MS m / z:390.2[M+H] + .
Embodiment 3
[0113] Example 3: 1-methyl-3-((2-((1-methyl-1H-pyrazol-4-yl)amino)-7H-pyrrole[2,3-d]pyrimidin-4-yl) Amino)pyridin-2(1H)-one
[0114]
[0115] 1 H NMR (600MHz, DMSO-d 6 )δ11.23(s,1H),8.82(s,1H),8.69(s,1H),8.22(s,1H),7.90(s,1H),7.50(s,1H),7.35(dd,J =6.7,1.4Hz,1H),6.92(dd,J=3.4,2.3Hz,1H),6.44(dd,J=3.3,1.8Hz,1H),6.31(t,J=7.1Hz,1H),3.80 (s,3H),3.57(s,3H).ESI-MS m / z:337.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com