Method for screening severe preeclampsia patient in early pregnancy
A technology for preeclampsia and early pregnancy, applied in the field of clinical medicine, can solve problems such as affecting fetal health, low accuracy of prediction indicators, and missed intervention opportunities, so as to reduce adverse pregnancy events, improve prediction accuracy, and reduce CVD exposure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
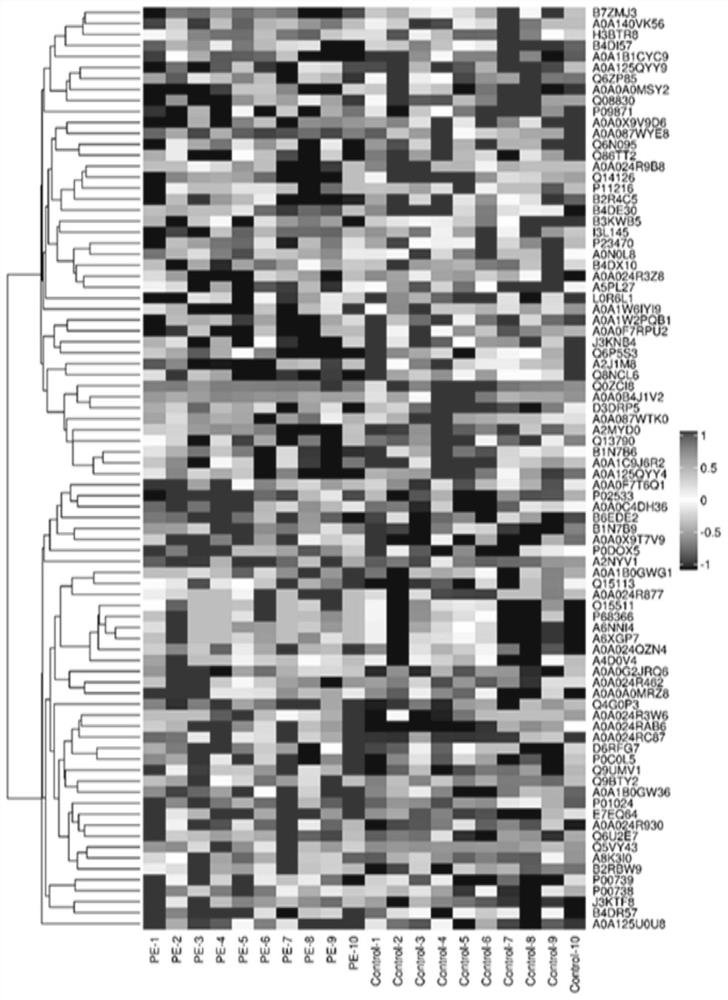
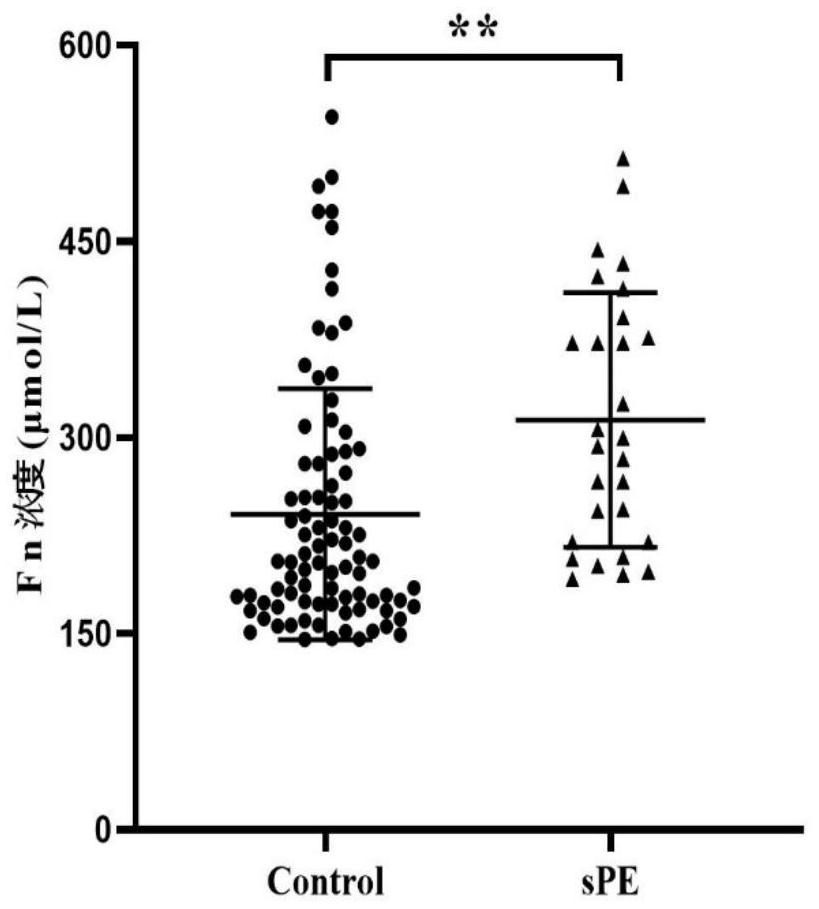
[0067] First trimester plasma fibronectin (FN) and complement 4b (C4b) can be used as the discovery process of sPE first trimester plasma markers, including the following steps:
[0068] 1. Since November 2016, 19 community hospitals in Tianjin, China have carried out community enrollment of singleton pregnant women in the first trimester, followed up to 45 days after the end of pregnancy, a prospective cohort study with HDP as the main entry point, and enrolled pregnant women All signed the written informed consent and the questionnaire. The purpose of the questionnaire was to collect the baseline characteristics of pregnant women, including age, ethnicity, education level, occupation, spouse status, family history, previous disease history, pregnancy parity, abnormal birth history, last time Menstruation, pre-pregnancy weight, pregnancy medications, lifestyle, etc., and corresponding physical examination and blood sample collection, and the maternal and child information syst...
Embodiment 2
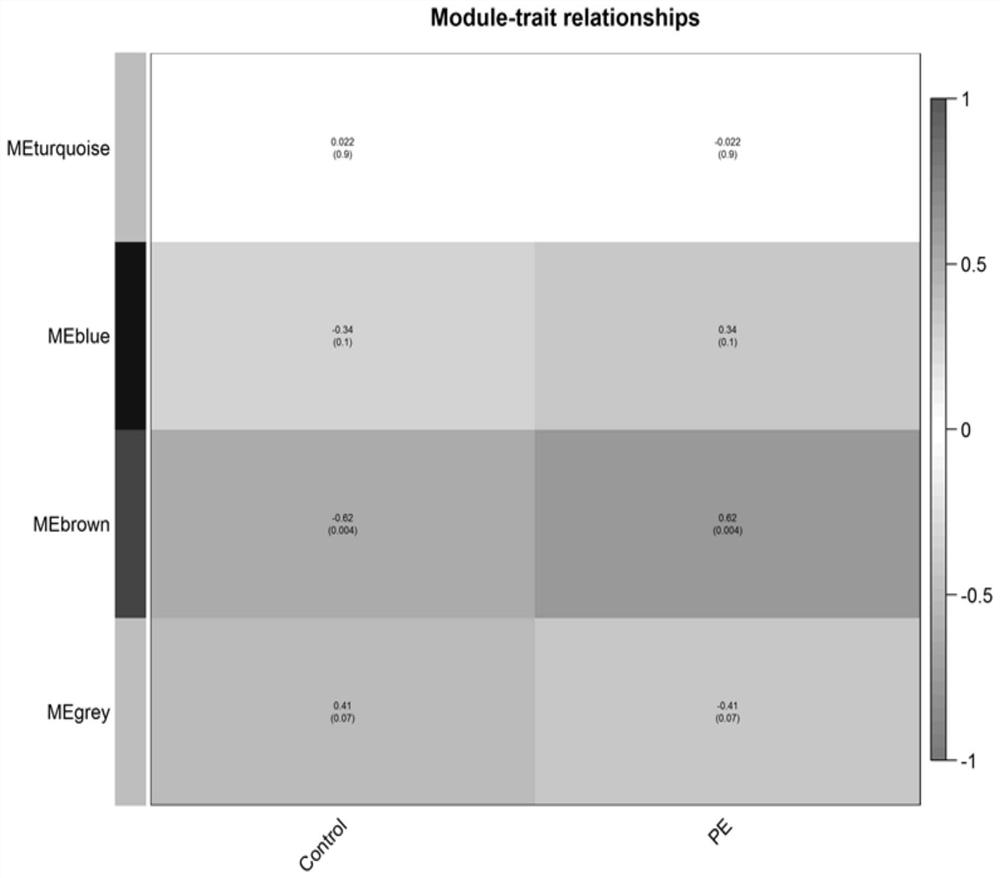
[0074] Predictive value of baseline characteristics (BMI, SBP) and plasma FN and C4b on sPE in pregnant women with sPE in the first trimester, and the sPE prediction model combined with the levels of FN and C4b in plasma biomarkers and the four indicators of BMI and SBP in baseline characteristics The comparison of (BMI+SBP+FN+C4b) accuracy with respect to the predictive model (BMI+SBP) of pregnant women's baseline characteristics includes the following steps:
[0075] 1. On the basis of the above-mentioned cohort, 29 pregnant women with sPE and their plasma samples in the first trimester were randomly selected, and 88 pregnant women with normal delivery and their plasma samples in the first trimester were randomly selected, and enzyme-linked immunosorbent assay (ELISA) was performed on the plasma samples ( Enzyme Linked Immunosorbent Assay, ELISA) detection. The human FN ELISA kit is the product of abcam company, article number ab108848, and the human C4b ELISA kit is the pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com