Preparation method of an iron-based nitrogen-phosphorus co-doped porous carbon-oxygen reduction catalyst
A co-doping and catalyst technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of complex process, poor stability, and low electrochemical performance, and achieve the effect of enhancing ORR performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
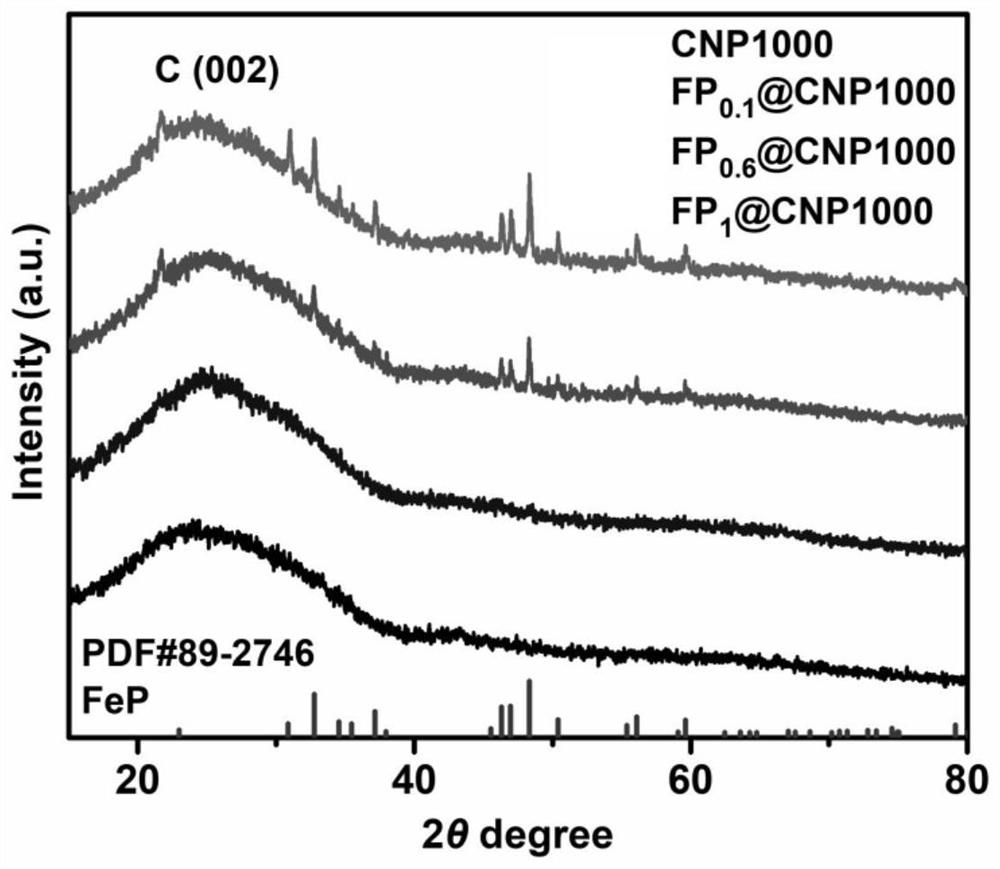
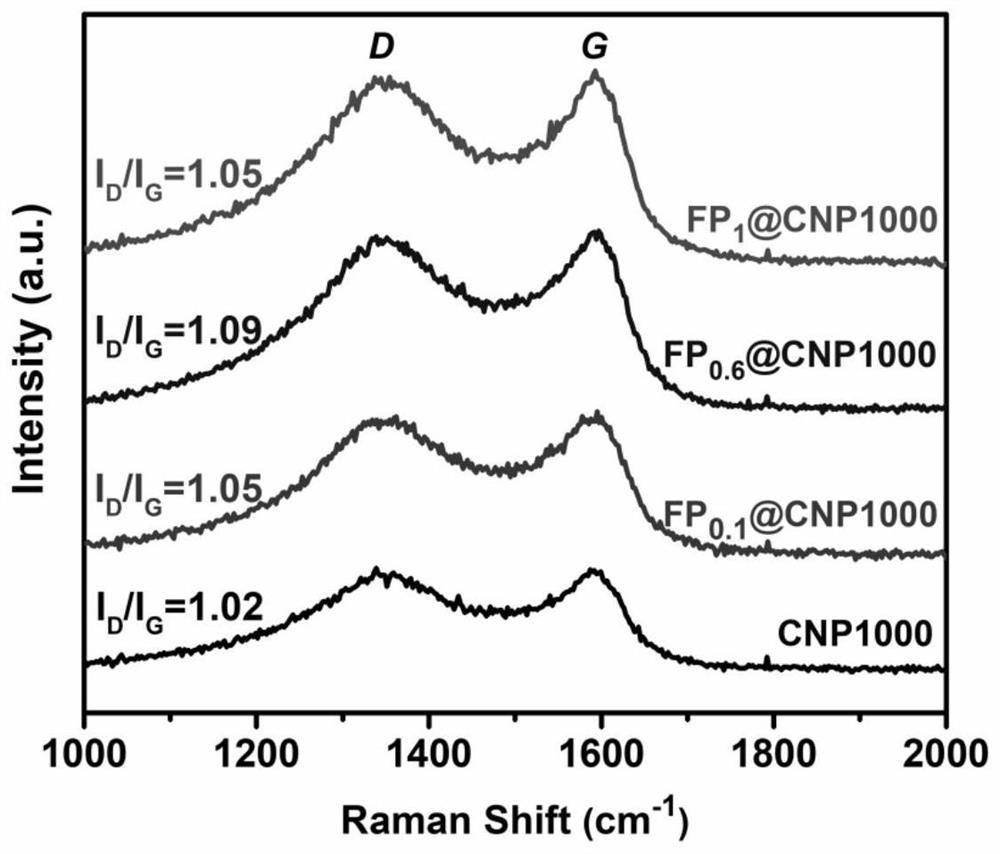
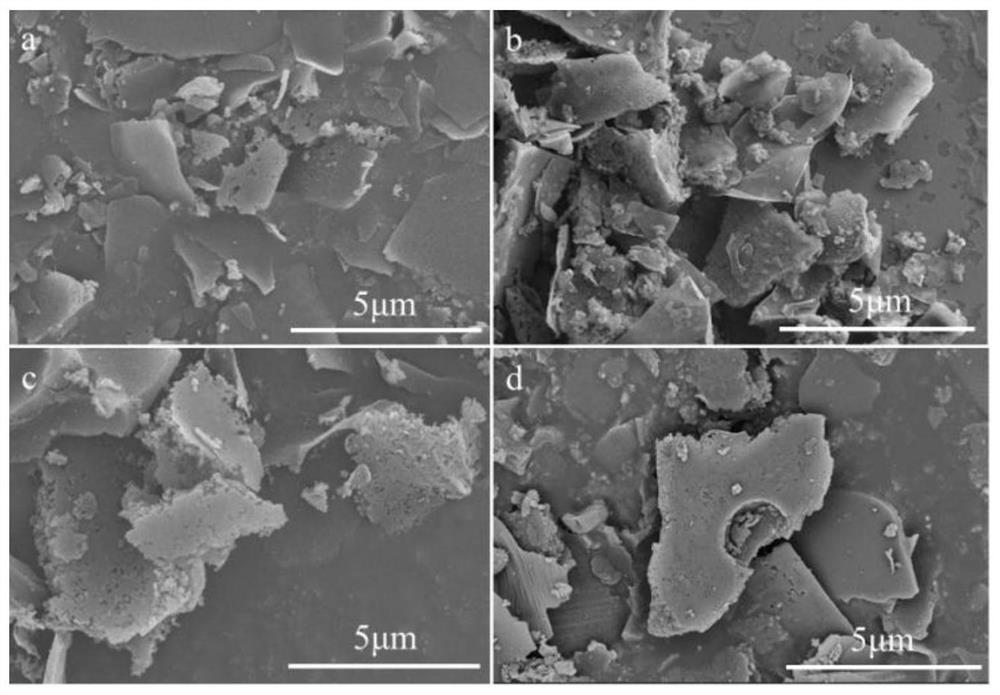
[0037] see Figure 1-Figure 6 , the present invention provides a preparation method and application of an iron-based nitrogen-phosphorus co-doped porous carbon ORR catalyst to solve the existing problems of complex process flow, poor stability and unsatisfactory electrochemical performance.
[0038] The present invention is specifically realized through the following technical solutions:
[0039] First, prepare a nitrogen-phosphorus co-doped nanosheet material (CNP1000), including the following steps:
[0040] Prepare a mixed solution containing urea, anhydrous glucose, phytic acid and deionized water; evaporate the mixed solution to dryness and put it into a vacuum oven to obtain the product; put the obtained product into a tube furnace for high-temperature carbonization treatment, and finally A nitrogen-phosphorus co-doped nanosheet material was obtained, which was named CNP1000.
[0041] The step of preparing the mixed solution containing urea, glucose anhydrous, phytic a...
Embodiment 1
[0049] A preparation method of nitrogen and phosphorus co-doped nano sheet material (CNP1000), comprising the following steps:
[0050] (1) Use an analytical balance to weigh 4g of urea and 400mg of anhydrous glucose into a 100mL beaker, use a pipette gun to add 1mL of phytic acid solution into the beaker, then add 40mL of deionized water to the beaker, and place the beaker Stir evenly on a magnetic stirrer.
[0051] (2) Evaporate the mixed solution prepared in step (1) at 80° C. until it is evaporated to dryness, and then put it into a vacuum oven and dry at 60° C. for 12 hours to obtain the product.
[0052] (3) Distribute the product prepared in step (2) evenly in an alumina dry pot, place the dry pot in a tubular high-temperature furnace, and continuously feed nitrogen gas at a rate of 15 mL / min at a rate of 5 °C / min. Raise the temperature to 1000°C. After heat preservation for 1 h, the temperature was lowered to 300 °C at a rate of 10 °C / min, and then the temperature wa...
Embodiment 2
[0054] An iron-based nitrogen-phosphorus co-doped nanosheet material (FP 0.1 @CNP1000), including the following steps:
[0055] (1) Weigh 0.1 mg of iron phthalocyanine into a 100 mL beaker with an analytical balance, add 50 mL of absolute ethanol into the beaker, and completely disperse the iron phthalocyanine in absolute ethanol.
[0056] (2) Weigh 25 mg of CNP1000 prepared in Example 1 and add it into a beaker. Place the beaker in an ultrasonic device and continue ultrasonication for 1 hour, then transfer the beaker to a stirring table, evaporate to dryness at 60°C, and form a cake-like black material at the bottom of the beaker. Then put the whole beaker into an oven and dry it at 60°C for 12 hours. The resulting product is iron-based nitrogen-phosphorus co-doped nanosheet material, named FP 0.1 @CNP1000.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com