Cobalt metaphosphate/nitrogen-carbon-oxygen reduction catalyst as well as preparation method and application thereof
A cobalt metaphosphate and catalyst technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of low initial potential and half-wave potential, and achieve the effect of simple method, enhanced ORR performance, and promotion of electrocatalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Accurately weigh 582.10 mg Co(NO 3 ) 2 ·6H 2 The O solid was dissolved in 20 mL of methanol solution, and then ultrasonically and magnetically stirred until a transparent pink cobalt nitrate methanol solution was obtained.
[0028] (2) Slowly drop 2 mL of ATMP aqueous solution with a mass concentration of 50 wt.% into the above-mentioned cobalt nitrate methanol solution drop by drop, react for 15 minutes to obtain a mixed solution, and then centrifuge, wash, and dry to obtain pink cobalt metaphosphate Coordination polymer precursors.
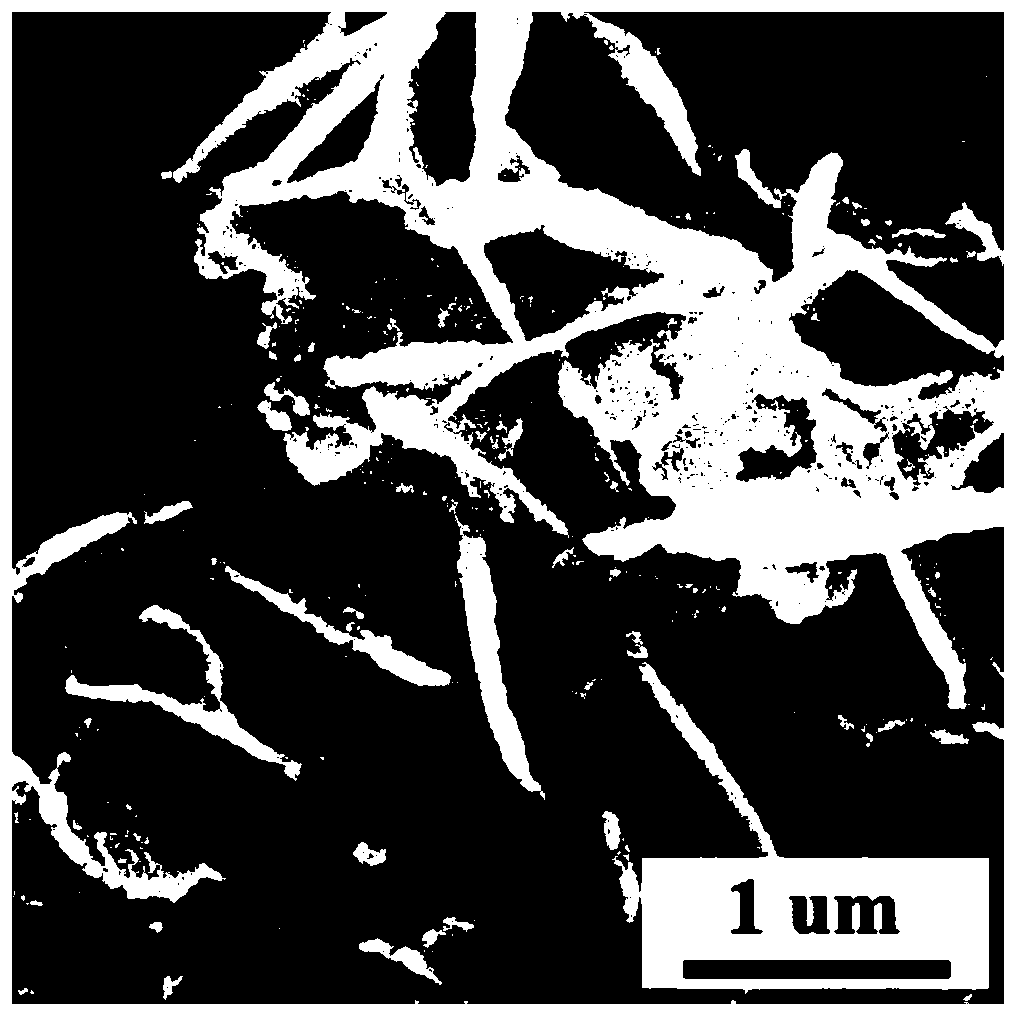
[0029] (3) The cobalt metaphosphate coordination polymer precursor formed in step 2 was placed in a tube furnace, and calcined at 700 °C for 1 h under a protective gas atmosphere to obtain a cobalt metaphosphate / nitrogen carbon oxygen reduction catalyst.
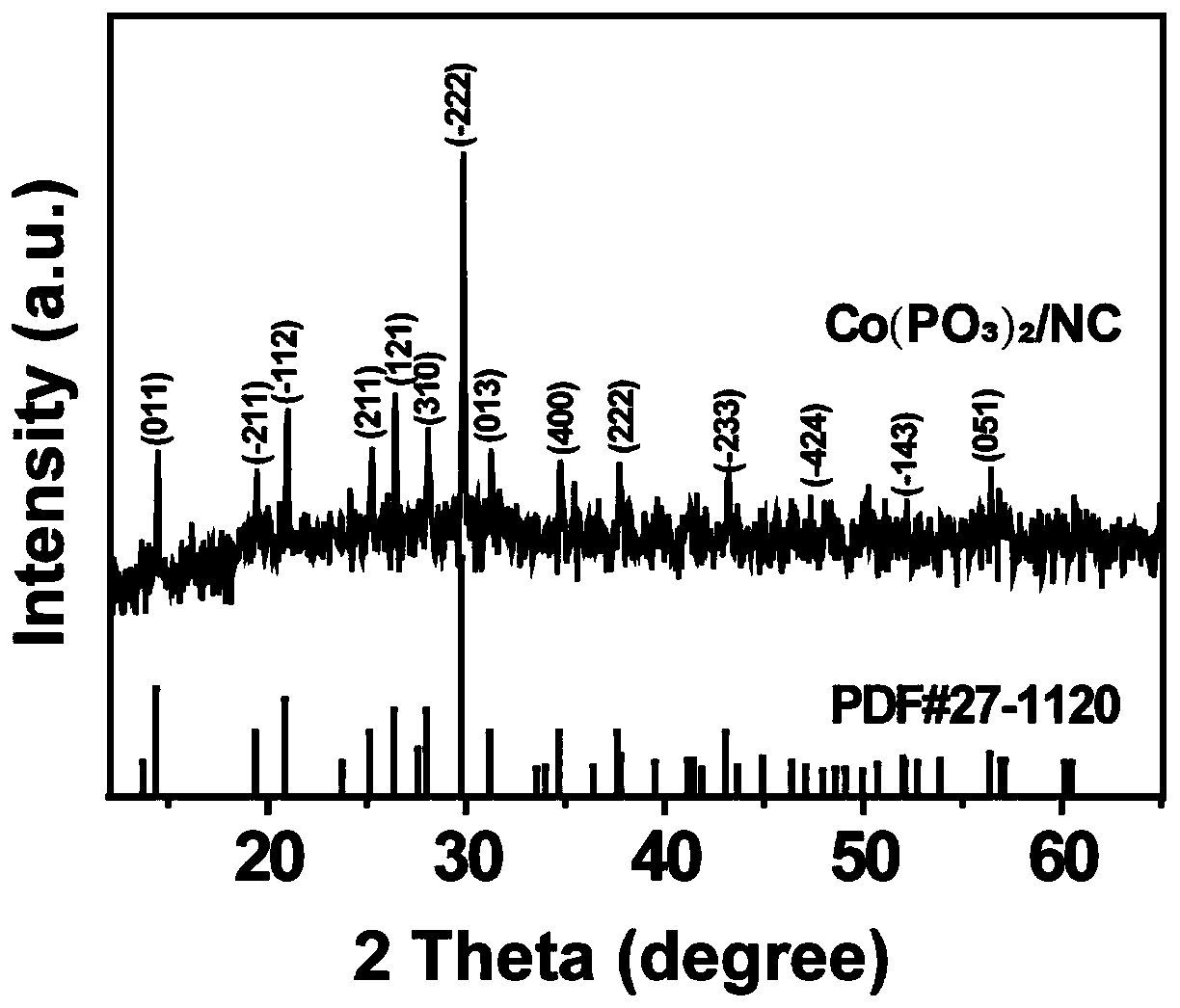
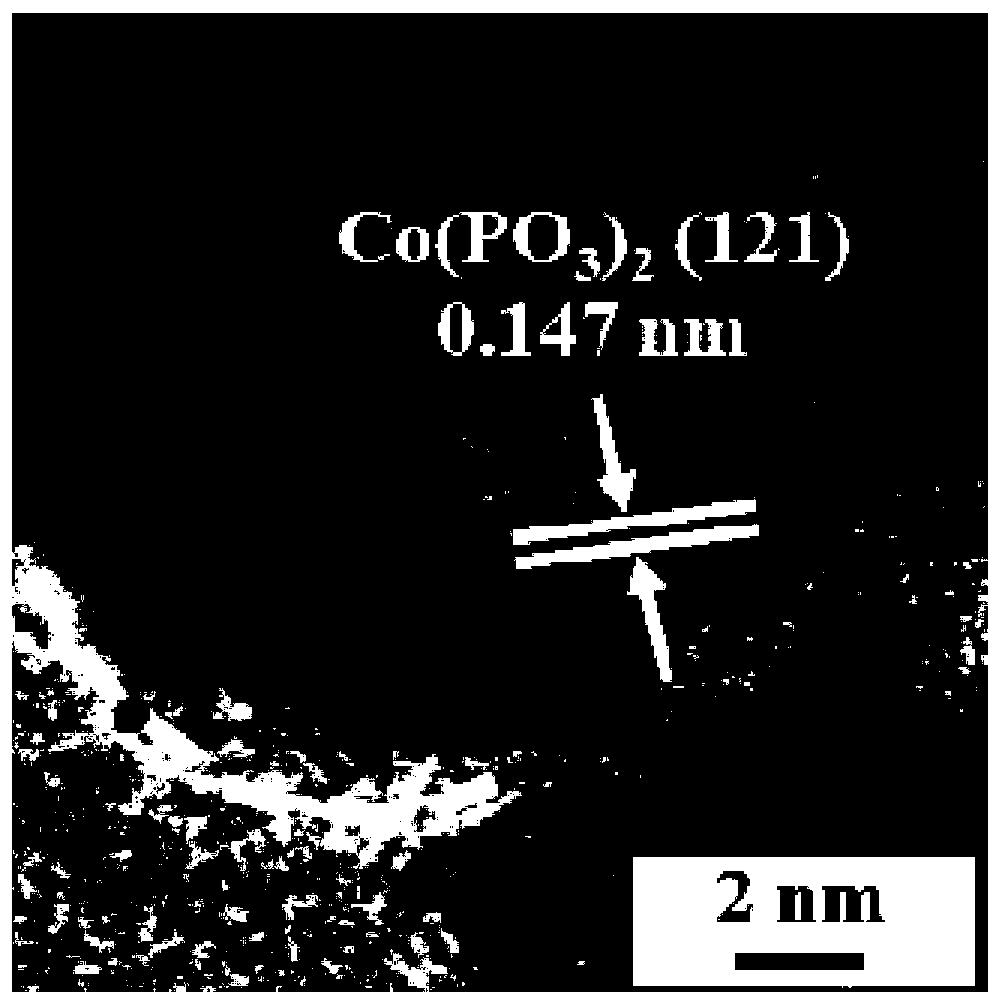
[0030] figure 1 According to the X-ray diffraction pattern of the product obtained in Example 1 of the present invention, all X-ray powder diffraction peaks can be indexed as Co...
Embodiment 2
[0032] (1) Accurately weigh 1164.2 mg Co(NO 3 ) 2 ·6H 2 The O solid was dissolved in 20 mL of methanol solution, and then ultrasonically and magnetically stirred until a transparent pink cobalt nitrate methanol solution was obtained.
[0033] (2) Slowly drop 2 mL of ATMP aqueous solution with a mass concentration of 55 wt.% into the above-mentioned cobalt nitrate methanol solution, and react for 30 minutes to obtain a mixed solution, which is then centrifuged, washed, and dried to obtain pink cobalt metaphosphate Coordination polymer precursors.
[0034] (3) The cobalt metaphosphate coordination polymer precursor formed in step 2 was placed in a tube furnace, and calcined at 800 °C for 1 h under a protective gas atmosphere to obtain a cobalt metaphosphate / nitrogen carbon oxygen reduction catalyst.
Embodiment 3
[0036](1) Accurately weigh 1746.18 mg Co(NO 3 ) 2 ·6H 2 The O solid was dissolved in 20 mL of methanol solution, and then ultrasonically and magnetically stirred until a transparent pink cobalt nitrate methanol solution was obtained.
[0037] (2) Slowly drop 2 mL of ATMP aqueous solution with a mass concentration of 60 wt.% into the above-mentioned cobalt nitrate methanol solution, and react for 60 minutes to obtain a mixed solution, which is then centrifuged, washed, and dried to obtain pink cobalt metaphosphate Coordination polymer precursors.
[0038] (3) The cobalt metaphosphate coordination polymer precursor formed in step 2 was placed in a tube furnace, and calcined at 900 °C for 2 h under a protective gas atmosphere to obtain a cobalt metaphosphate / nitrogen carbon oxygen reduction catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com