Application of tetrahydrocannabivarinic in prevention and/or treatment of pulmonary arterial hypertension
A technology of tetrahydrocannabinol and pulmonary arterial hypertension, applied in the field of medicine, can solve problems such as the lack of a cure for pulmonary arterial hypertension, the uncurable mortality rate of pulmonary arterial hypertension, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] 1. Experimental animals, reagents and instruments
[0090] (1) Experimental animals
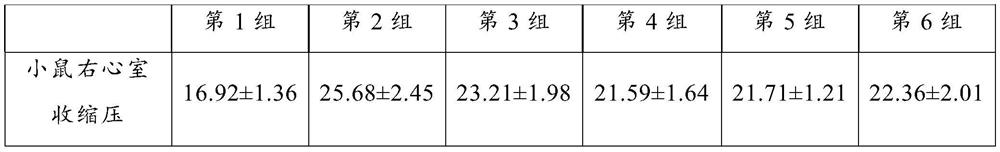
[0091] 4-6 weeks old mice, healthy and active, with shiny coat color, weighing (25.15±2.15) g, C57BL / 6 (Experimental Animal Center of Military Science and Medical College, SPF grade). Group the experimental animals as follows:
[0092] Group 1 (normoxic wild type, as a control group): under normoxia, 10 female mice and 10 male mice;
[0093] Group 2 (anoxic wild type, as a control group): under hypoxic conditions, 10 female mice and 10 male mice;
[0094] Group 3 (THCV administration group, 0.2 mg / kg body weight): intragastric administration under hypoxic conditions, 10 female mice and 10 male mice;
[0095] Group 4 (THCV administration group, 0.4mg / kg body weight): intragastric administration under hypoxic conditions, 10 female mice and 10 male mice;
[0096] Group 5 (THCV administration group, 0.8 mg / kg body weight): intragastric administration under hypoxic conditions, 10 female...
Embodiment 2
[0158] With reference to the relevant parameters of Example 1, the experimental mice were taken and grouped as follows:
[0159] Group 1 (combined administration group THCV 0.4mg / kg body weight + CBD 10mg / kg body weight): intragastric administration under hypoxic conditions, 10 female mice and 10 male mice;
[0160] Group 2 (combined administration group THCV 0.4mg / kg body weight + CBDV 2mg / kg body weight): intragastric administration under hypoxic conditions, 10 female mice and 10 male mice;
[0161] Group 3 (combined administration group THCV 0.4 mg / kg body weight + CBG 0.5 mg / kg body weight): intragastric administration under hypoxic conditions, 10 female rats and 10 male rats.
[0162] Group 4 (combined administration group THCV 0.4 mg / kg body weight + CBD 10 mg / kg body weight + CBDV 2 mg / kg body weight): intragastric administration under hypoxic conditions, 10 female mice and 10 male mice;
[0163] Group 5 (combined administration group THCV 0.4mg / kg body weight+CBD 10mg...
Embodiment 3
[0175] Take industrial hemp flowers and leaves and grind them, pass through No. 1 sieve, bake at 100°C for 200 minutes, take a certain amount of roasted cannabis flowers and leaves medicinal materials, add 70% ethanol according to the material-to-liquid ratio of 1:8 (w / v), and stir at room temperature Extract twice, 1 hour each time, filter, combine the extracts, centrifugal filter, concentrate the centrifugal filtrate under reduced pressure (65°C, -0.08~-0.09Mpa) until it has no alcohol smell and the density is 1.070 (60°C), add Purify the water until the weight of the solution is 8 times the weight of the medicinal materials, and stir evenly to obtain the sample solution.
[0176] Measure the sample solution, purify it through a treated macroporous resin column (diameter-to-height ratio: 1:5) at a flow rate of 4BV / h, let it stand for 60 minutes after loading, and then use purified water at a flow rate of 9BV / h , 50% ethanol, 80% ethanol elution, use molish reaction to determ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com