Anti-tigit antibodies and uses thereof
An antibody and antigen technology, applied in the field of biology, can solve the problems of drug resistance and low response rate of immunotherapy, and achieve the effect of promoting activation, enhancing anti-tumor immune response, and promising drug prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1: Preparation of anti-TIGIT antibody mouse monoclonal antibody
[0124] This example describes the preparation of mouse anti-human TIGIT monoclonal antibodies using hybridoma technology. First, the TIGIT protein in the extracellular region (Uniprot: Q495A1) was expressed as an immunogen, and a 6xHis tag was added to the C-terminal of the amino acid sequence of the TIGIT protein in the extracellular region (Met22-Pro141), and then cloned into an expression vector to obtain the TIGIT protein in the extracellular region. The expression vector was transiently transfected into 293 cells (source: ATCC), and the cell culture fluid was collected and purified 5 days later. To prepare anti-human TIGIT mouse monoclonal antibody, 4-week-old BAL B / c mice (provided by Viton Lever) were firstly immunized with 100 μg of TIGIT protein in the extracellular region. On the 14th and 28th days after the first immunization, the immunized mice were re-immunized with 50 μg of TIGIT pr...
Embodiment 2
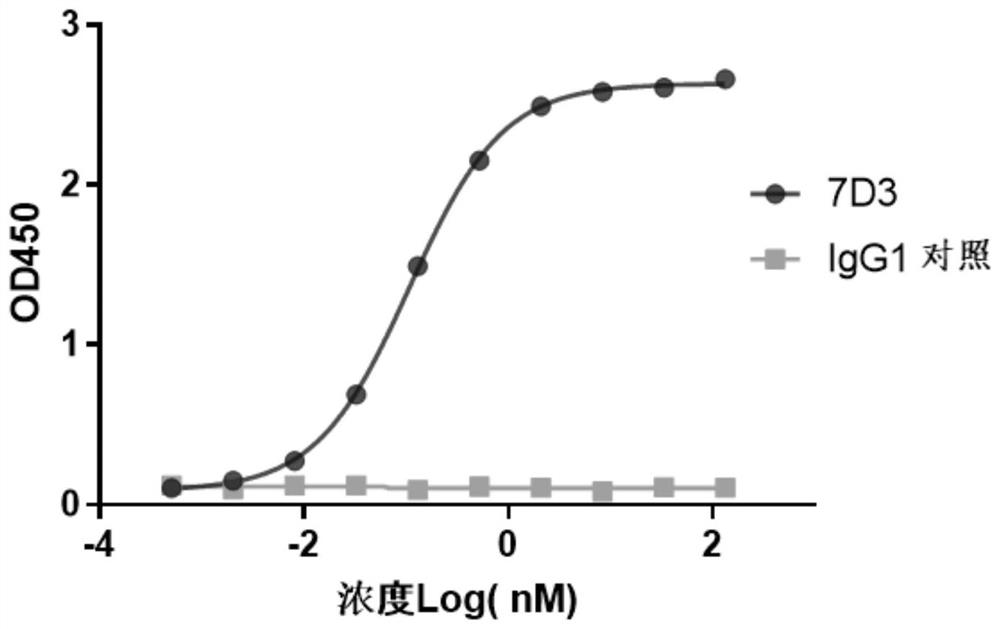
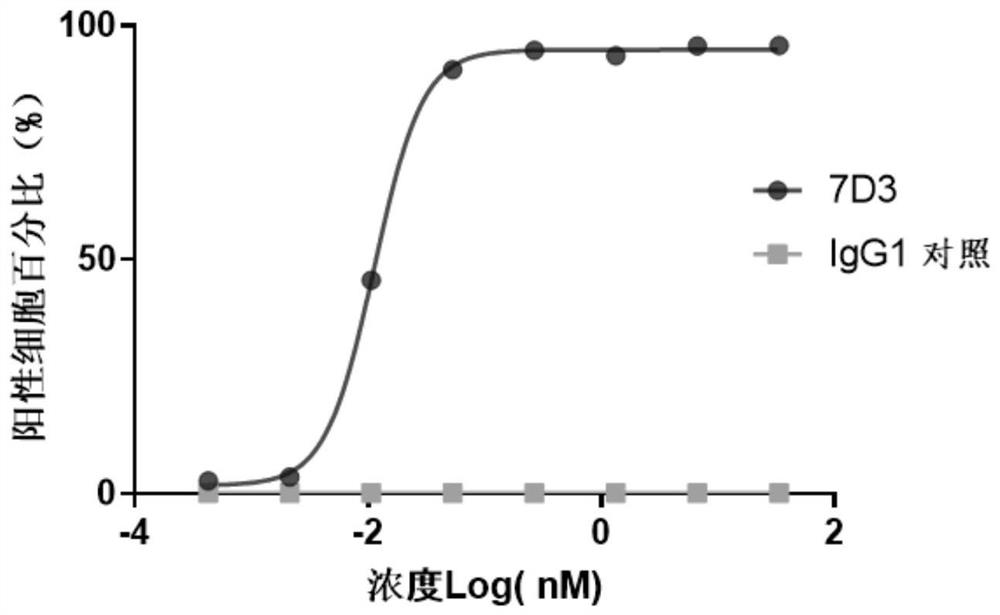
[0130] Example 2: Binding of murine antibodies to TIGIT
[0131]The binding ability of mouse-derived antibody to human TIGIT protein was quantitatively detected by ELISA. Human TIGIT protein (manufacturer: Acrobiosystems, product number: TIT-H52H3) was diluted into PBS at a concentration of 0.5 μg / ml, and coated on a microplate at 4°C. overnight. Then blocked with 5% BSA-PBST blocking solution at 37°C for 1h; after washing the plate with PBST, 7D3 was diluted to different concentrations (133.33nM starting, 4 times dilution, a total of 10 concentrations), added to the plate and incubated at 37°C for 1 hour. Wash the plate, add goat anti-mouse IgG (manufacturer: Sigma, product number: A2554, 1:5000 dilution), and react at 37°C for 1 hour. After washing the plate, add TMB solution, react in the dark at room temperature for 10 minutes, and then add 2N H 2 SO 4 Terminate the reaction. Placed on a microplate reader to detect absorbance at 450 nm wavelength. see the results fig...
Embodiment 3
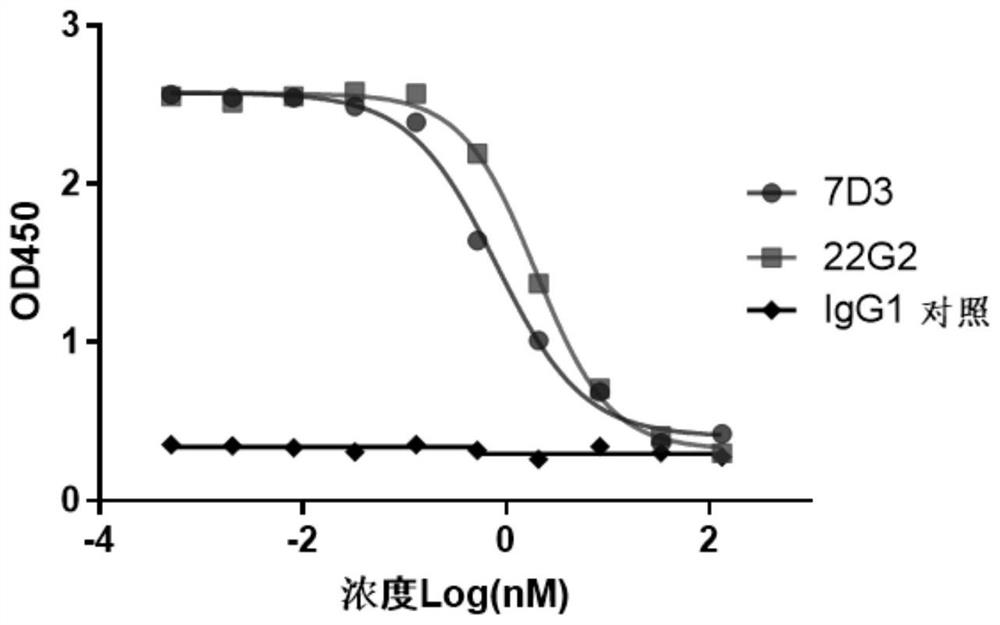
[0133] Example 3: Blocking effect of murine anti-TIGIT antibody on binding of TIGIT to CD155
[0134] The blocking effect of anti-TIGIT antibody 7D3 on the binding of TIGIT to CD155 was detected by the competitive ELISA method described in Example 1. The initial concentration of the antibody was 133.33 nM, and 4-fold gradient dilution was performed with 0.2% BSA-PBST to obtain a total of 10 gradients. Using 22G2 as a positive control, according to the sequences disclosed in US10189902 (the variable region sequences of heavy and light chains are SEQ ID NO: 7, SEQ ID NO: 9, respectively, and the constant region sequences are SEQ ID NO: 45, SEQ ID NO: 49, respectively) 22G2. The result of the comparison is image 3 The IC50 of 7D3 is 0.804nM, and the IC50 of 22G2 is 1.961nM, which shows that 7D3 can effectively block the binding of TIGIT to CD155, and the blocking effect of 7D3 antibody on TIGIT and CD155 is stronger than that of 22G2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com