Preparation method of SGLT2 inhibitor intermediate
A technology of inhibitors and intermediates, which is applied in the field of preparation of SGLT2 inhibitor intermediates, can solve the problems of unsuitability for industrial application and production, more time required, and small reactor volume, etc., and achieve easy recycling, simple treatment, The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
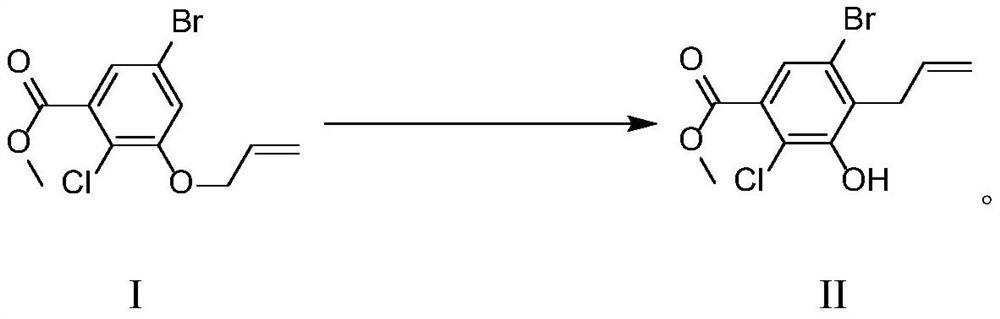
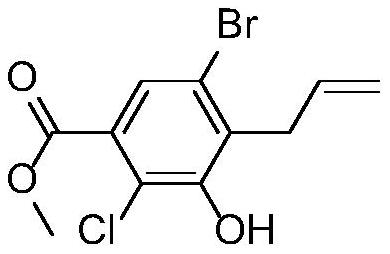
[0033] 1) Preparation of raw material solution: Stir and mix 500g (1.636mol) of methyl 3-(allyloxy)-5-bromo-2-chlorobenzoate and 250g of diphenyl ether, and connect to metering pump P1;
[0034] 2) Set the continuous flow reactor I, the circulation temperature is 220°C, and reach stability; set the continuous flow reactor II, the circulation temperature is 40°C, and reach stability.
[0035] 3) Add 1500 g of n-heptane into the collection kettle, and install a mechanical stirring device.
[0036] 4) Set the flow rate of metering pump P1 to 50.0ml / min, turn on metering pump P1, run in reactor I for 5 minutes, and run in reactor II for 10 minutes, then collect the reaction solution.
[0037] 5) After pumping all the raw materials, the collected reaction solution was cooled to 5 degrees Celsius, stirred for 1 hour, filtered, and dried to obtain 475 g of the target product, with a yield of 95% and an HPLC of 98%.
Embodiment 2
[0039] 1) Preparation of raw material solution: Stir and mix 500g (1.636mol) of methyl 3-(allyloxy)-5-bromo-2-chlorobenzoate and 250g of simethicone oil, and connect to metering pump P1;
[0040] 2) Set the continuous flow reactor I, the circulation temperature is 220°C, and reach stability; set the continuous flow reactor II, the circulation temperature is 40°C, and reach stability.
[0041] 3) Add 1500 g of n-heptane into the receiving container, and install a mechanical stirring device.
[0042] 4) Set the flow rate of metering pump P1 to 50.0ml / min, turn on metering pump P1, run in reactor I for 5 minutes, and run in reactor II for 10 minutes, then collect the reaction solution.
[0043] 5) After pumping all the raw materials, the collected reaction solution was cooled to 5 degrees Celsius, stirred for 1 hour, filtered, and dried to obtain 465 g of the target product, with a yield of 93% and an HPLC of 98%.
Embodiment 3
[0045] 1) Preparation of raw material liquid: Stir and mix 500g (1.636mol) of methyl 3-(allyloxy)-5-bromo-2-chlorobenzoate and 250g of paraffin oil, and connect to metering pump P1;
[0046] 2) Set the continuous flow reactor I, the circulation temperature is 220°C, and reach stability; set the continuous flow reactor II, the circulation temperature is 40°C, and reach stability.
[0047]3) Add 1500 g of n-heptane into the receiving container, and install a mechanical stirring device.
[0048] 4) Set the flow rate of metering pump P1 to 50.0ml / min, turn on metering pump P1, run in reactor I for 5 minutes, and run in reactor II for 10 minutes, then collect the reaction solution.
[0049] 5) After pumping all the raw materials, cool the collected reaction solution to 5 degrees Celsius, stir for 1 hour, filter, and dry to obtain 480 g of the target product, with a yield of 96% and an HPLC of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com