2-nitroglycal and efficient synthesis method thereof
A synthesis method and technology of nitrosaccharene, which is applied in the field of 2-nitrosaccharene and its high-efficiency synthesis, can solve the problems of strong electron donating ability, large steric hindrance, and failure to obtain nitrosaccharene products, and achieve simple operation, good selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The technical solution of the present invention will be described in further detail below in conjunction with the examples, but the protection scope of the present invention is not limited thereto.
[0047]
[0048] Table 1
[0049]
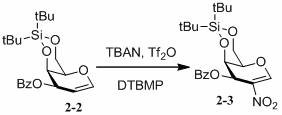
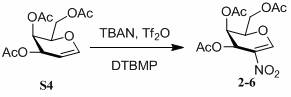
[0050] Comprehensive screening of the above conditions, TBAN (1.4eq), Tf 2 O (1.4eq), DTBMP (2eq), dichloromethane as solvent, reaction conditions at -70°C.
[0051]
[0052] Table 2
[0053]
[0054]
[0055] Based on the above conditions, TBAN(2eq), Tf 2 O (2eq), DTBMP (4eq), reaction conditions at -30°C.
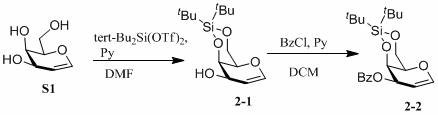
[0056] Synthesis of glucoene raw materials:
[0057]
[0058] For the specific preparation process of S4 to S5 and S9 to S1, please refer to V.D. Bussolo, M. Caselli, M. Pineschi, P. Crotti, Org. Lett. 2003, 5, 2173-2176.
[0059]
[0060] Compound S5 (2g, 13.7mmol) was dissolved in 30mL DMF, imidazole (1.8639g, 27.4mmol, 2eq) was added, TBDPSCl (4mL, 15.1mmol, 1.1eq) was added at 0°C, N 2 Reaction under pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com