Synthesis of benzenesulfonamide phenylbutyric acid podophyllotoxin ester derivatives and application of benzenesulfonamide phenylbutyric acid podophyllotoxin ester derivatives in anti-cancer drugs
A technology of benzenesulfonamide phenylbutyric acid and podophyllotoxin, which is applied in the field of preparation of benzenesulfonamide phenylbutyric acid podophyllotoxin carboxylate derivatives, can solve problems such as toxic and side effects, limit clinical use and the like, and achieve toxic and side effects Low, obvious tumor cell inhibitory activity, strong inhibitory activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Example 1: Preparation of Formula I Benzenesulfonamide Benzenebutyrate Podophyllotoxin Ester Derivatives
[0026] Dissolve 5.58 mmol of p-aminophenylbutyric acid in 20 mL of water, stir at room temperature, adjust the pH to 8-9 with saturated sodium carbonate solution, and continue stirring until the acid is completely dissolved in the water. To the reaction system, 6 mmol of benzenesulfonyl chloride containing different substituents was added, and the mixture was stirred at room temperature overnight. After the reaction finishes, dropwise add the hydrochloric acid solution with a concentration of 1 mol / L to the reaction system to a pH of 1~2, separate out a large amount of solid, then use a Buchner funnel through vacuum filtration, wash with water, and dry the light yellow obtained. Solid, the intermediate product benzenesulfonamide phenylacetic acid derivative is obtained. This product can be used directly in the next chemical synthesis, or it can be further purified...
example 2
[0045] Example 2: Application of Formula I Benzenesulfonamide Benzenebutyrate Podophyllotoxin Ester Derivatives
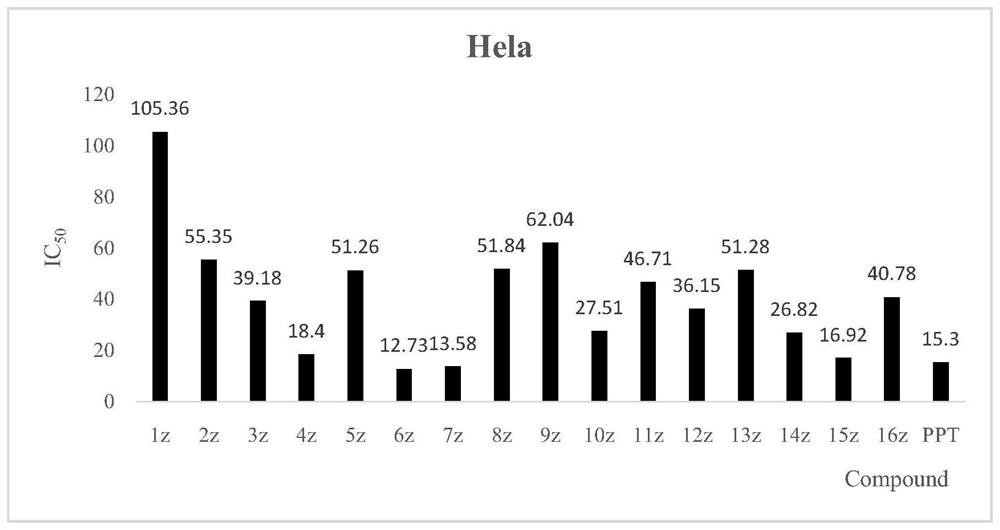
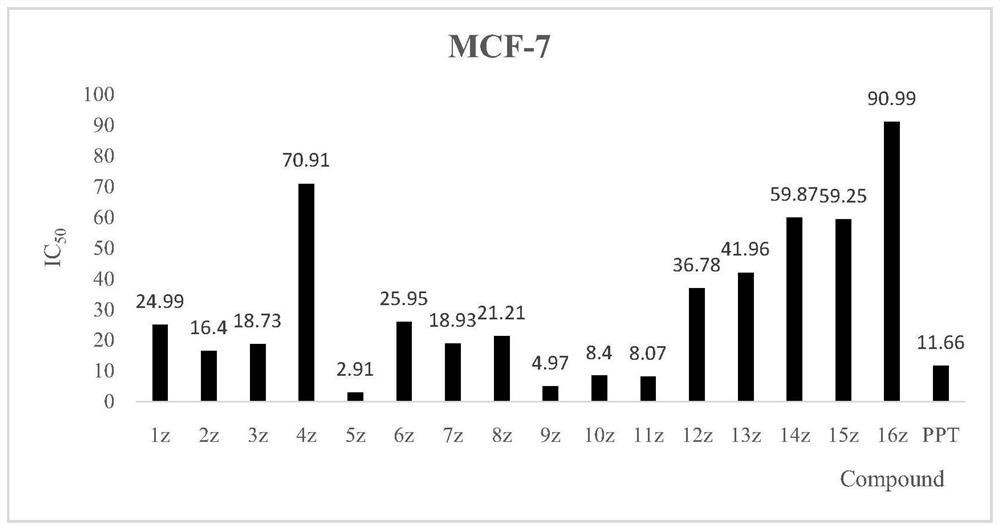
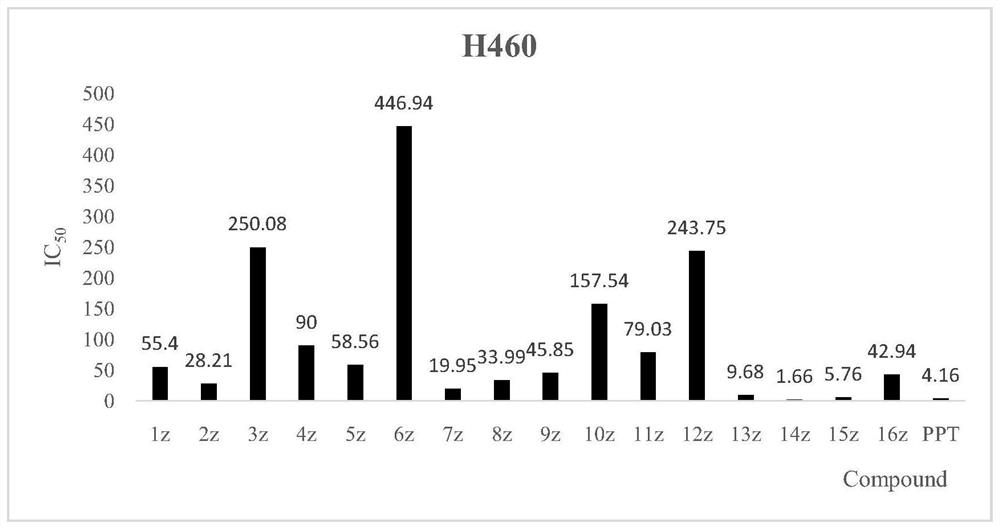
[0046] MCF-7, HeLa, H460, A549 and L02 human normal liver cell lines were used as detection strains, and MTT colorimetry was used as detection method. The inhibitory activity study found that the novel structural derivatives have obvious tumor cell inhibitory activity in vitro. The results are attached figure 1 , 2 , 3, 4.
example 3
[0047] Example 3: Compound 5z significantly induces apoptosis
[0048] Different concentrations (0, 0.5, 1, 2 μM) of compound 5z and PPT (2 μM) acted on A549 cells respectively, treated for 24 h, collected cells, centrifuged, and washed cells twice with PBS; The cells were resuspended in bindingbuffer, 5 μL of FITC and 10 μL of LPI were added, and the cells were stained in the dark for 15 minutes, and cell apoptosis was detected by flow cytometry. The results are attached Image 6 . The derivatives of this novel structure can obviously promote the apoptosis of human non-small cell lung cancer cell A549.
[0049] The benzenesulfonamide phenylbutyrate podophyllotoxin ester derivatives of the present invention can be prepared into antitumor drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com