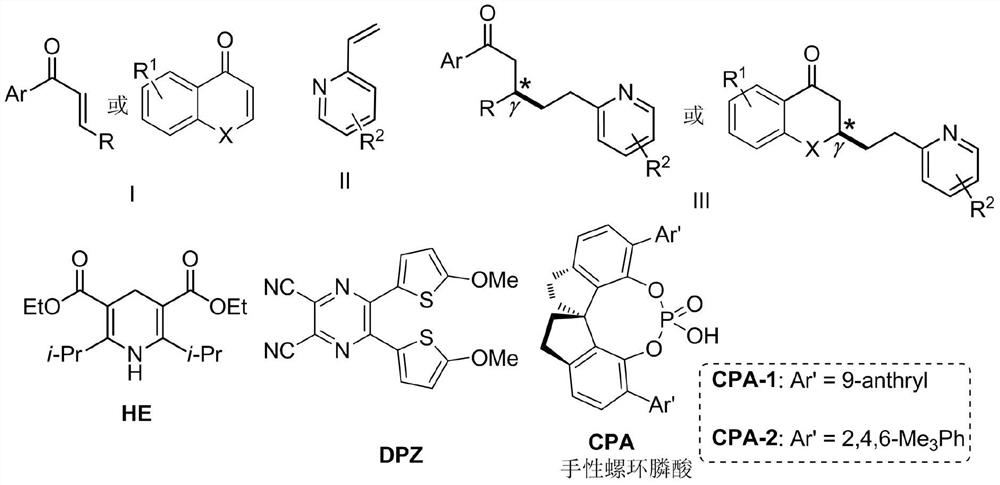
A visible-light asymmetric catalyzed olefin cross-coupling method to construct pyridine derivatives containing γ-chiral centers
A chiral center and cross-coupling technology, applied in organic chemistry methods, organic chemistry, etc., can solve problems such as unrealized, and achieve the effects of good selectivity, mild reaction conditions, and less catalyst consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
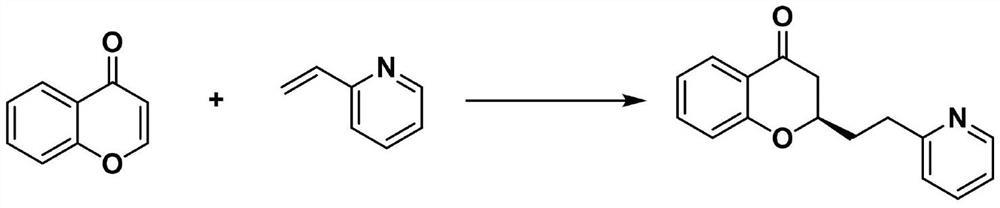
[0019] A method for the construction of a γ-chiral center-containing pyridine derivative by asymmetric catalyzed olefin cross-coupling with visible light, the reaction formula is as follows. The specific preparation steps of (R)-2-(2-(pyridin-2-yl)ethyl)chroman-4-one are as follows:
[0020]
[0021]35.5 μL (0.0005 mmol, 0.005 equiv) of a solution of DPZ (prepared with 1.0 mg of DPZ in 200 uL toluene) was added to a 25 mL Schlenk tube and the solvent was removed in vacuo. Subsequently, 21.9 mg (0.15 mmol) of 4H-chroman-4-one, 10.5 mg (0.10 mmol) of 2-vinylpyridine, 113.3 mg (0.02 mmol) of chiral spirocyclic phosphonic acid, and 55.6 mg (0.02 mmol) of chiral spirophosphonic acid were added. 0.18mmol), then 2 ml of purified and dried dichloromethane were added, followed by vacuuming, freezing at -80°C for 3-5min, returning to room temperature, and argon protection (generally the entire process was carried out three times), the reaction flask was placed in - In a 25°C incubat...
Embodiment 2
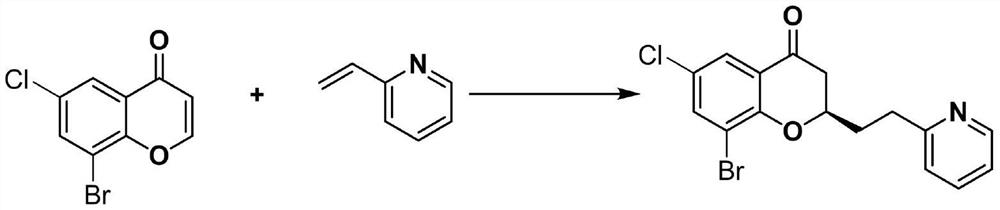
[0023] The reaction formula for the synthesis of (R)-8-bromo-6-chloro-2-(2-(pyridin-2-yl)ethyl)chroman-4-one is shown below.
[0024]
[0025] In this example, the 4H-chroman-4-one in Example 1 was replaced with 8-bromo-6-chloro-4H-chroman-4-one, and other steps were the same as in Example 1 to obtain (R) -8-Bromo-6-chloro-2-(2-(pyridin-2-yl)ethyl)chroman-4-one 22.8 mg as yellow oil, yield 62%, optical purity 95% ee. The NMR data are: 1 H NMR (300MHz, CDCl 3 )δ8.50(d, J=4.1Hz, 1H), 7.64(m, 3H), 7.21(d, J=7.7Hz, 1H), 7.09(m, 1H), 4.47(td, J=12.6, 8.6 Hz, 1H), 3.08(m, 2H), 2.72(d, J=7.3Hz, 2H), 2.28(m, 2H); 13 C NMR (75MHz, CDCl 3 )δ190.2, 160.1, 156.4, 149.3, 138.2, 136.4, 126.8, 125.6, 123.2, 122.3, 121.3, 112.6, 77.9, 42.3, 34.0, 33.2; high-resolution data: HRMS(ESI)m / z 365.9894(M+H + ),calc.for C 16 H 164 NO 2 BrCl365.9891.
Embodiment 3
[0027] The reaction formula for the synthesis of (R)-6-methyl-2-(2-(pyridin-2-yl)ethyl)chroman-4-one is shown below.
[0028]
[0029] In this example, 4H-chroman-4-one in Example 1 was replaced with 6-methyl-4H-chroman-4-one, and other steps were the same as in Example 1 to obtain (R)-6- Methyl-2-(2-(pyridin-2-yl)ethyl)chroman-4-one 16.3 mg, yellow oil, yield 61%, optical purity 92% ee. The NMR data are: 1 H NMR (300MHz, CDCl 3 )δ8.22(d,J=4.3Hz,1H),7.30(dd,J=16.3,8.6Hz,2H),6.97(d,J=9.0Hz,1H),6.88(d,J=7.8Hz, 1H), 6.81(m, 1H), 6.57(d, J=8.4Hz, 1H), 4.14(dt, J=8.9, 4.9Hz, 1H), 2.74(m, 2H), 2.39(m, 2H), 1.92(m,5H); 13 C NMR (75MHz, CDCl 3 )δ192.6, 160.7, 159.6, 149.4, 137.0, 136.5, 130.6, 126.5, 123.0, 121.3, 120.6, 117.7, 43.0, 34.5, 33.5, 20.4; high-resolution data: HRMS(ESI)m / z268.1333(M+ H + ),calc.for C 17 H 18 NO 2 268.1332.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com