Interleukin 2 binding molecule, and derivative, kit, production method and use of interleukin 2 binding molecule
A technology of interleukin and binding molecules, which is applied in the field of single-domain antibodies against interleukin 2, can solve the problem of fewer antibodies and achieve high affinity, high stability, and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
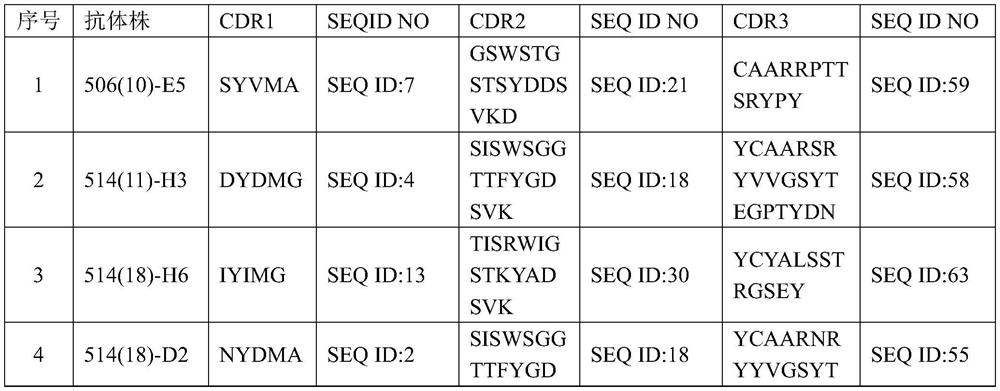
[0064] This embodiment discloses an interleukin 2 binding molecule, which can specifically bind interleukin 2, and comprises at least one immunoglobulin single variable domain, and at least one immunoglobulin single variable domain comprises CDR1, CDR2 and CDR3. wherein CDR1 comprises an amino acid sequence at least 90% identical to any one of SEQ ID NOs: 1-15, CDR2 comprises an amino acid sequence at least 90% identical to any one of SEQ ID NOs: 16-54, and CDR3 comprises an amino acid sequence identical to any one of SEQ ID NOs: 16-54. An amino acid sequence that is at least 90% identical to any one of SEQ ID NOs: 55-84. In this embodiment at least one immunoglobulin single variable domain comprises CDR1, CDR2 and CDR3 shown in Table 1 below.
[0065] Table 1 CDR1, CDR2 and CDR3 of interleukin-2 binding molecules
[0066]
[0067]
[0068]
[0069]
[0070]
[0071] Among them, the immunoglobulin single variable domain is VHH. The VHH comprises amino acid se...
Embodiment 2
[0076] This example discloses the screening production process of Example 1, and the 54 antibody strains obtained through screening are subjected to nucleotide sequencing to obtain nucleic acid sequences.
[0077] 1. Material preparation: Prepare the commercial materials shown in Table 3 below.
[0078] table 3
[0079]
[0080] 2. Immunization
[0081] To induce a humoral immune response against IL2 in camelids, alpacas were injected subcutaneously with 5 doses of human IL2 protein at 1 to 3 week intervals. The dosage range is 150ug to 500ug per injection.
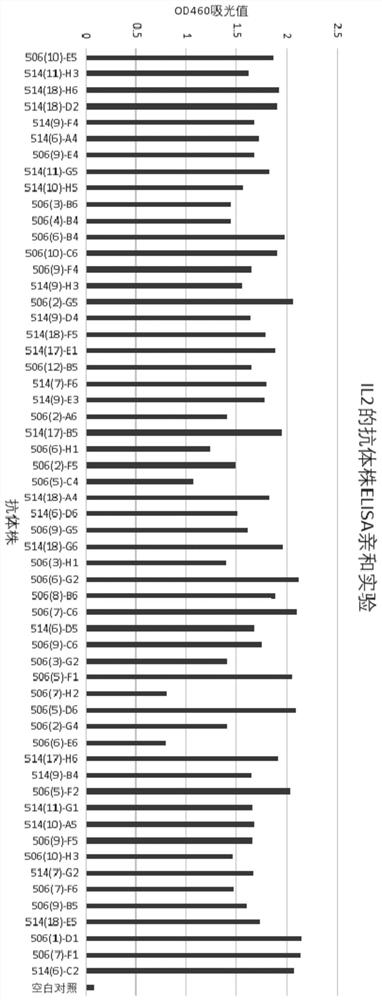
[0082] 3. Serum titer detection
[0083] After immunization, serum titers of anti-IL2 specific antibodies were determined by ELISA. For ELISA experiments, ELISA (NEST, 504201) was coated with 400 ng / well recombinant his-tagged human IL2 protein and incubated overnight at 4°C. After blocking and washing, serially diluted preimmune and immune sera were added and incubated at room temperature for 2 hours, then incuba...
Embodiment 3
[0114] This embodiment discloses an immunoconjugate and a pharmaceutical composition.
[0115] The immunoconjugate comprises a therapeutic moiety and the interleukin-2 binding molecule in Example 1. Therapeutic moieties include cytotoxins, biologically active proteins or radioisotopes. The therapeutic moiety is conjugated to the interleukin-2 binding molecule.
[0116] The pharmaceutical composition comprises the interleukin-2 binding molecule in Example 1 or the above immunoconjugate, and a pharmaceutically acceptable carrier.
[0117] The pharmaceutical composition is a drug for preventing or treating a proliferative disorder, a disease or condition that may respond to an IL2 antagonist: such as a drug for treating cancer, or a drug for treating chronic viral infection, or a drug for inhibiting or blocking the binding of CD25 and interleukin 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com