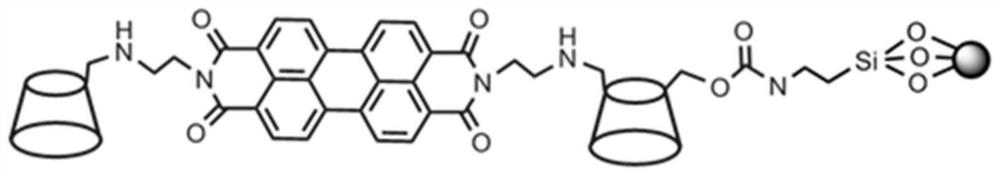
Perylene diimide bridged double β-cyclodextrin stationary phase and its preparation method and application
A technology of perylene diimide-based bridge and cyclodextrin, which is applied in the field of chiral separation materials, can solve the problem of small improvement in stationary phase selectivity, unfavorable cyclodextrin inclusion effect, difficult to control quantity and site, etc. problem, to achieve good separation effect, improve synergistic inclusion and chiral separation ability, and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of perylene diimide bridged double β-cyclodextrin stationary phase, comprising the following steps:
[0027] (1) Mix 1.7mmol 6-monoethylenediamine-β-cyclodextrin, 0.7mmol perylenetetracarboxylic dianhydride, 1.0mmol anhydrous zinc acetate and 120mL pyridine, stir to form a homogeneous solution, and under nitrogen protection, React in an oil bath at 100°C for 48 hours, then distill off the solvent under reduced pressure, add 100mL of water to dissolve the solid residue, filter, collect the filtrate, concentrate the filtrate to 1 / 3 of the original volume, add methanol to produce a large amount of precipitation, suction filter, vacuum the solid After drying, a purple-red product (perylene diimide bridged bis-cyclodextrin) was obtained;
[0028] (2) Dissolve the bridged bis-β-cyclodextrin synthesized above in 40 mL of anhydrous N,N-dimethylformamide (DMF) under magnetic stirring, and slowly add 3-isocyanato group at room temperature Propyltriethoxysila...
Embodiment 2
[0034] A preparation method of perylene diimide bridged double β-cyclodextrin stationary phase, comprising the following steps:
[0035] (1) Mix 2.0mmol 6-monoethylenediamine-β-cyclodextrin, 1.0mmol perylenetetracarboxylic dianhydride, 1.0mmol anhydrous zinc acetate and 120mL pyridine, stir to form a homogeneous solution, and under nitrogen protection, React in an oil bath at 100°C for 48 hours, then distill off the solvent under reduced pressure, add 100mL of water to dissolve the solid residue, filter, collect the filtrate, concentrate the filtrate to 1 / 3 of the original volume, add methanol to produce a large amount of precipitation, suction filter, vacuum the solid After drying, a purple-red product (perylene diimide bridged bis-cyclodextrin) was obtained;
[0036] (2) Dissolve the bridged bis-β-cyclodextrin synthesized above in 40 mL of anhydrous N,N-dimethylformamide (DMF) under magnetic stirring, and slowly add 3-isocyanato group at room temperature Propyltriethoxysila...
Embodiment 3
[0042] A preparation method of perylene diimide bridged double β-cyclodextrin stationary phase, comprising the following steps:
[0043] (1) Mix 2.3mmol 6-monoethylenediamine-β-cyclodextrin, 1.3mmol perylenetetracarboxylic dianhydride, 1.0mmol anhydrous zinc acetate and 120mL pyridine, stir to form a homogeneous solution, and under nitrogen protection, React in an oil bath at 100°C for 48 hours, then distill off the solvent under reduced pressure, add 100mL of water to dissolve the solid residue, filter, collect the filtrate, concentrate the filtrate to 1 / 3 of the original volume, add methanol to produce a large amount of precipitation, suction filter, vacuum the solid After drying, a purple-red product (perylene diimide bridged bis-cyclodextrin) was obtained;
[0044] (2) Dissolve the bridged bis-β-cyclodextrin synthesized above in 40 mL of anhydrous N,N-dimethylformamide (DMF) under magnetic stirring, and slowly add 3-isocyanato group at room temperature Propyltriethoxysila...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com