Method for synthesizing UDP-galactose and galactosyl compound
A technology of galactose and galactokinase, which is applied in the field of bioengineering and can solve problems such as unseen galactosyl compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: TTHA0595, TTE0732 and TON_1857 Engineering The construction of bacteria and purification of proteins, including the following steps:
[0024] 1. TTHA0595, TTE0732 and TON_1857 gene amplification
[0025] The primer sequences of TTHA0595, TTE0732 and TON_1857 gene amplification are as follows:
[0026] (1) Thermophilic hemodalose kinase TTHA0595:
[0027] Upstream primers: atgaat Catatg Atgggcttccaagaggttttac, the horizontal line is the restriction NdeI identification site;
[0028] Downstream primer: aatccc Gaattc Ttagaggaccttgagg, horizontal line is restriction enzyme EcoRI identification site;
[0029] (2) Thermophilic UDP-glucose phosphorylation enzyme TTE0732:
[0030] Upstream primers: agcggaagagagggaaag Catatg AAAATAA, the horizontal line is the restriction enzyme NdeI identification site;
[0031] Downstream primer: cgccgccctctct Gaattc TTACACATCT, the horizontal line is the restriction enzyme EcoRI identification site;
[0032] (3) Iophilic preheating seq...
Embodiment 2
[0041] Example 2: Preparation of UDP-galactose
[0042] UDP-galactose synthesis includes two-step reaction, the specific process is as follows:
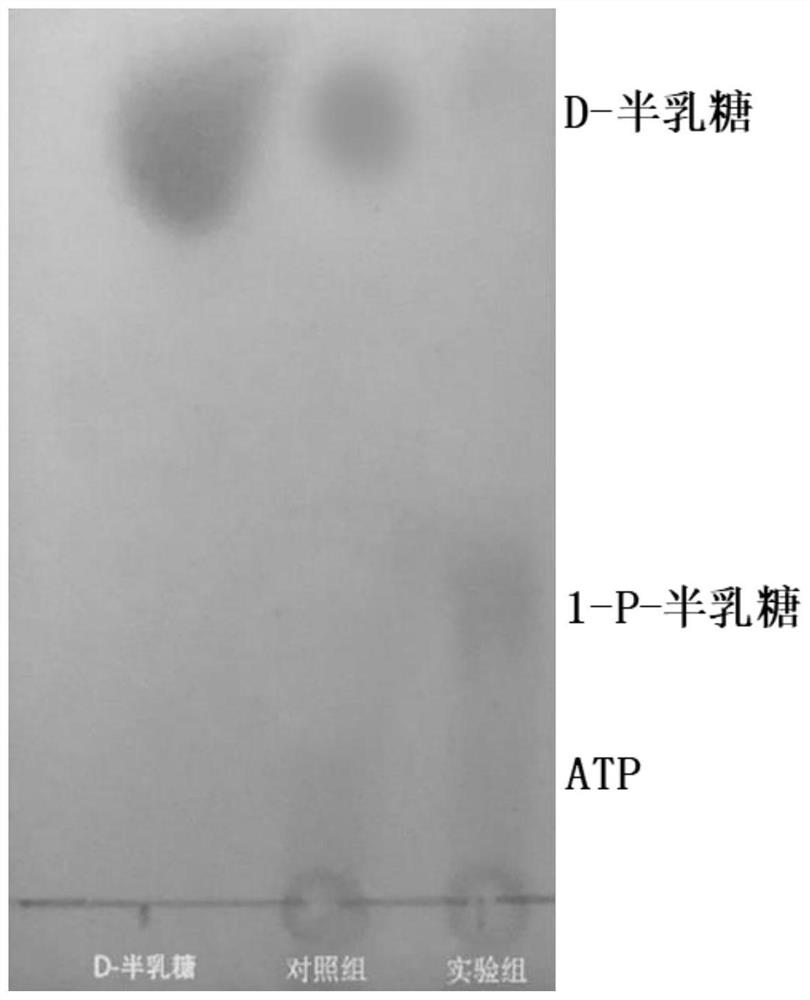
[0043] First step reaction: thermophilic galactose TTHA0595 catalyzed 1-p-GAL synthesis
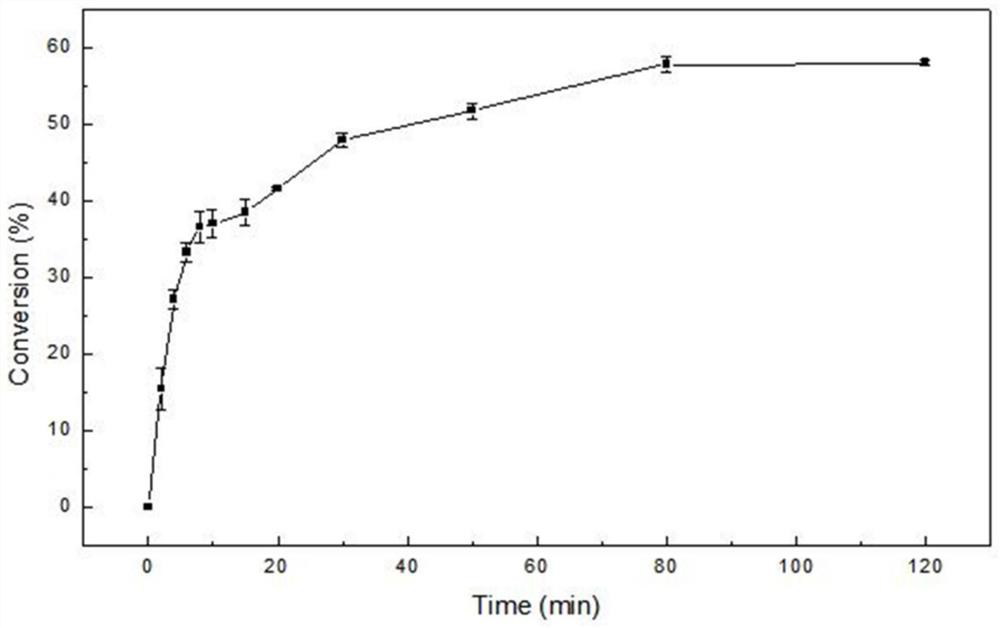
[0044] The galactose kinase is a phosphate donor by a molecular ATP (adenosine thiophosphate) to catalyzes D-galactose to form 1-p-gal and ADP (adenosine dithonic acid), and the reaction system is shown in Table 2. The reaction buffer is 20 mM Caps (3-cycloisopropathulfonic acid) aqueous solution (pH 8.0), sequentially adding the substrate D-galactose and ATP to a final concentration of 150 mm, then add MGCL 2 The final concentration was 5 mM, 50 ° C, and the thermophilic semicondrin kinase TTHA0595 was added to a final concentration of 0.4 mg / mL, and the revolution of the revolution of 150 rpm at 50 ° C was 0 to 120 min. figure 2 ). The experimental results show that the reaction is in 80min to achieve a balance, and the amount of product in 60 t...
Embodiment 3
[0055] Example 3: Synthesis of galactoselated compounds
[0056] Example 3 UDP-galactose synthesized in Example 2 was galactose-based donor, which catalyzed the syrogl-based alkyl group using the thermophilic preheating sequetolatum-based transferase TON_1857, and realized the compound fragrant laceanol, p-nitrophenol and water. The glycosylation modification of Jobin, the reaction system is shown in Table 4.
[0057] Table 4: TON_1857 Catalyzed the reaction raw material table of synthetic galactose compound
[0058]
[0059] The reaction buffer is 20 mM Caps aqueous solution (pH 9.0), and UDP-galactose having a final concentration of 5 mm, MGCl 2 And the fragrant lanterol or the nitrophenol or water fly Bin, mixed with a mixture of 5 min at 50 ° C, and then add a final concentration of 0.4 mg / ml of thermophilic preheating sequrites Ton_1857 at 50 ° C to 150 rpm / The number of revolutions of MIN reacted for 5h.
[0060] After the end of the reaction, it was boiled for 1 min i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com