In situ gene editing
A genome and coding technology, applied in gene therapy, genetic engineering, plant genetic improvement, etc., can solve problems such as large transplant failure, infection risk, and limited applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
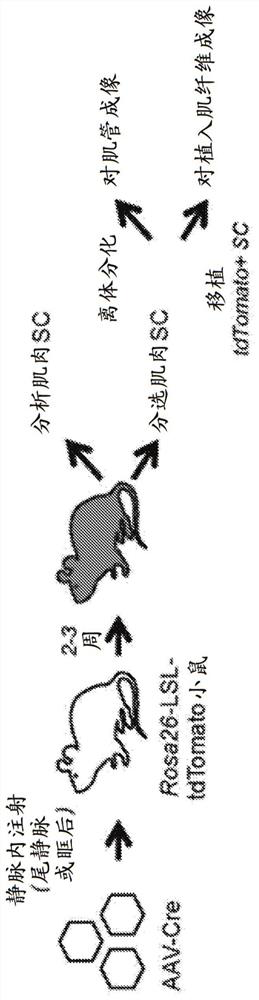
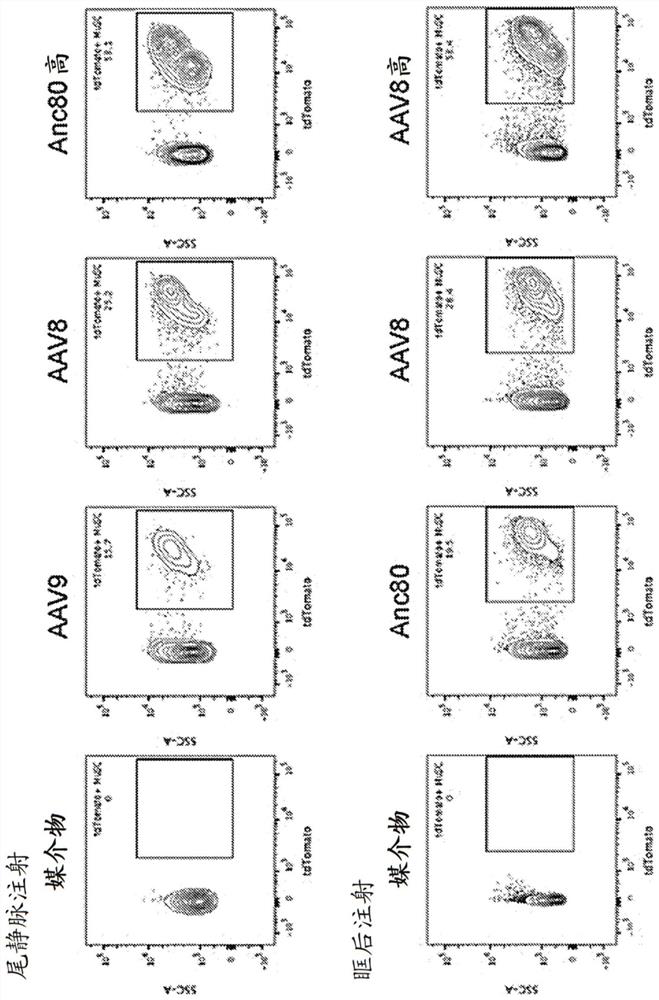
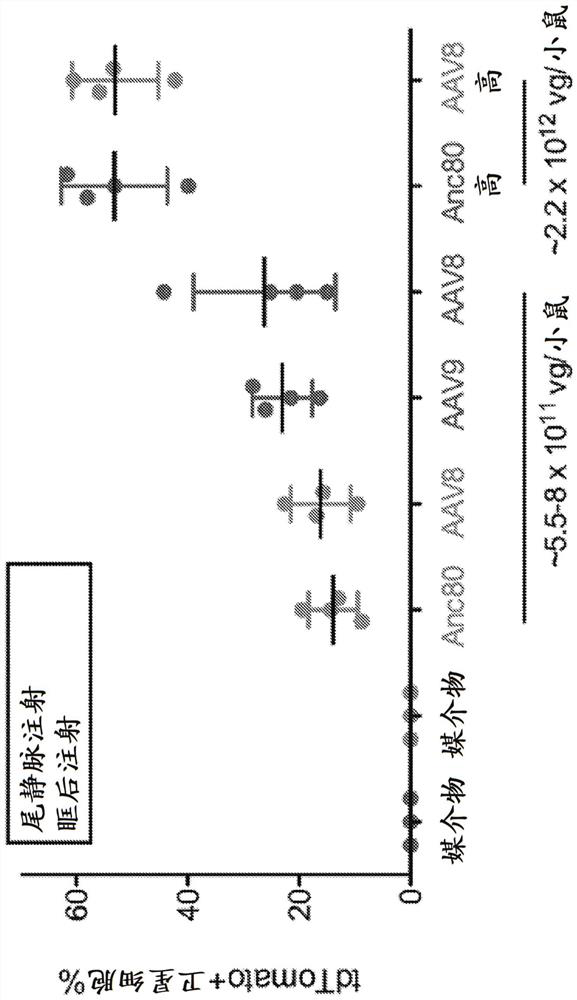
[0120] In vivo delivery of genome-modifying enzymes holds important promise for therapeutic applications and functional genetic screening. Of particular interest in this context is the delivery to endogenous tissue stem cells that provide a durable source of cell replacement in homeostasis and in response to regenerative cues. Here, a sensitive fluorescent reporter system activated by Cre recombinase was used to test the efficiency of genome modification following in vivo transduction of endogenous tissue stem cells by adeno-associated virus (AAV). We combined immunophenotyping with robust in vitro and in vivo stem cell functional assays to reveal efficient targeting of tissue-localized skeletal muscle satellite cells, mesenchymal progenitor cells, and hematopoietic stem cells using multiple AAV serotypes. The genome modification rate achieved by this method was as high as 65%, and the modified cells retained key functional properties. This study establishes a powerful new pl...
Embodiment 2
[0251] AAV carrying S. aureus Cas9 (saCas9) + gRNA targeting Dnmt3a or a control locus (Jak2) was injected into 2-month-old C57BL / 6 mice. After four months, liver and bone marrow were harvested from one mouse in each treatment group. Hematopoietic stem cells (HSC) and multipotent progenitor cells (MPP) were isolated from bone marrow by FACS. In addition, genomic DNA at the Dnmt3a locus was amplified and sequenced from each of these populations / organs (liver, bone marrow, HSC, and MPP).
[0252] result
[0253] Figure 11A Shown is the percentage of edited reads, ie, the percentage of sequenced reads containing indels in the amplified Dnmt3a sequence containing the gRNA-targeted site. Figure 11B Shown is the percentage of %WT reads, ie, the percentage of sequencing reads that map to the wild-type Dnmt3a sequence. Such as Figure 11A As shown, Dnmt3a-targeting gRNAs introduced about 30% of the Dnmt3a locus edits in the liver. Indels were also detected in ~0.5% of reads in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com