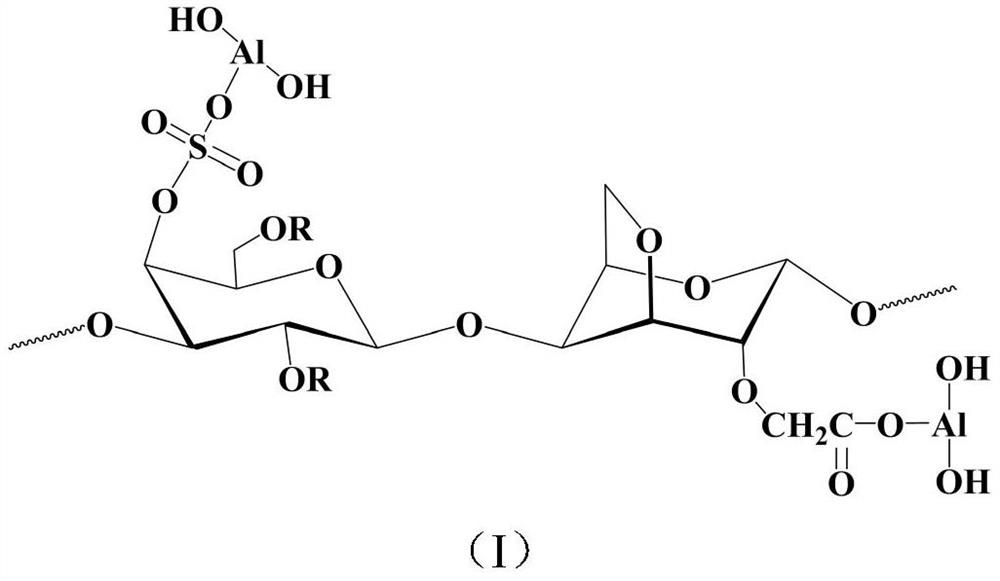
Carboxymethylated carrageenan-aluminum compound micro-hydrogel carrier
A technology of carboxymethylated carrageenan and compound, applied in the field of medical materials, can solve the problems of poor biodegradation, residue, long disintegration time, etc., and achieve the effect of reducing side effect time and side effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Preparation of Carboxymethylated Carrageenan
[0022] The κ-carrageenan powder was hydrated at 85°C for 15 min and overnight at room temperature to form a hydrated κ-carrageenan with a concentration of 5% (w / v). React at 10°C for 2.5h, gradually heat to 80°C, stir for 2h, stop the reaction, wash with methanol / water (80 / 20, v / v), adjust to neutral with glacial acetic acid, filter out the product, wash with methanol, And dry at 30-40°C.
Embodiment 1
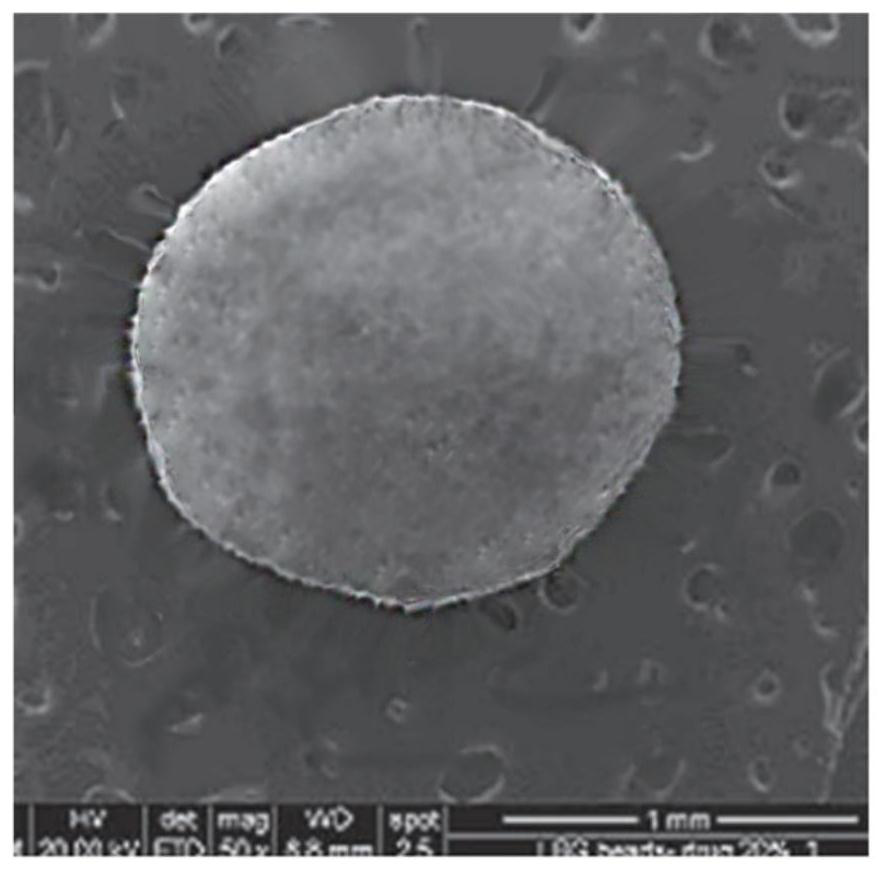
[0024] 25% (w / v) model drug diclofenac sodium was dissolved in 30mL carboxymethylated carrageenan aqueous solution, and the solution was squeezed into the concentration of 12, 14, 16, 18, 20% (w / v) (marked as 1-1, 1-2, 1-3, 1-4, 1-5) containing 100mL of five groups of basic aluminum chloride with different concentrations of 0.05% (w / v) Tween 80 solution, in which the basic aluminum chloride solution consists of AlCl 3 ·6H 2 O aqueous solution and 7% (w / v) ammonia water were reacted at 60-70°C, and the obtained droplets were incubated in the gel medium for 30min, and the micro-hydrogel particles were collected by filtration, and the excess was washed with distilled water. Surface salt or ion 2-3 times, air dry;
[0025] Carry out drug load rate (%) to above-mentioned five groups of samples, the complete release time of medicine is analyzed, and its measuring condition is as follows:
[0026] 1) Drug loading rate (%): Pulverize a known amount of micro-hydrogel particles (~10...
Embodiment 2
[0034] 25% (w / v) model drug diclofenac sodium was dissolved in 30mL carboxymethylated carrageenan aqueous solution, and the solution was squeezed into 18% (w / v) containing 0.05% ( w / v) in the basic aluminum chloride solution of 100mL of Tween 80 (1-4 of embodiment 1), wherein the basic aluminum chloride solution is made of AlCl 3 ·6H 2 O aqueous solution was reacted with 7% (w / v) ammonia water at 60-70°C, and the obtained droplets were incubated in the gel medium for 10, 20, 30, 40, 50, 60 min (denoted as 2- 1, 2-2, 2-3, 2-4, 2-5, 2-6), and collect micro-hydrogel particles by filtration, wash excess surface salt or ions with distilled water for 2 to 3 times, and air dry;
[0035] Carry out drug loading rate (%) to above-mentioned five groups of samples, the complete release time of medicine is analyzed, and its measuring condition is the same as embodiment 1, and the result obtained is shown in Table 2;
[0036] Table 2: Effect of droplet incubation time on drug loading rate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com